A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISRY
RESONANCE|Exercise Exercise 3|7 VideosELECTROCHEMISRY
RESONANCE|Exercise Part 2|23 VideosELECTROCHEMISRY
RESONANCE|Exercise Objective Questions|41 VideosELECTRO CHEMISTRY
RESONANCE|Exercise PHYSICAL CHEMITRY (ELECTROCHEMISTRY)|53 VideosEQUIVALENT CONCEPT & TITRATIONS
RESONANCE|Exercise Part -IV|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-ELECTROCHEMISRY-Comprehension
- Given: E(Zn^(+2)//Zn)^(@) =- 0.76V E(Cu^(+2)//Cu)^(@) = +0.34V K...
Text Solution
|
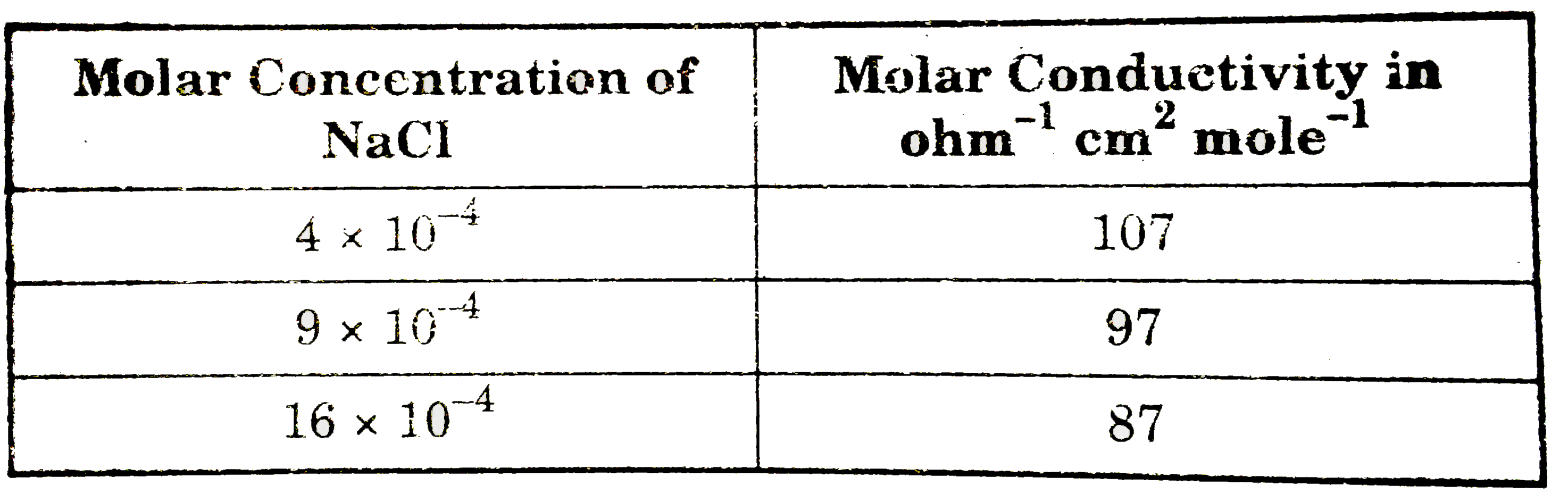
- The molar conductance of NaCl varies with the concentration as shown i...
Text Solution
|
- The molar conductance of NaCl varies with the concentration as shown i...
Text Solution
|
- The molar conductance of NzCl varies with the concentration as shown i...
Text Solution
|
- Strong acid versus strong base: The principle of conductometric titr...
Text Solution
|
- Strong acid versus strong base: The principle of conductometric titr...
Text Solution
|
- Strong acid versus strong base: The principle of conductometric titr...
Text Solution
|
- Tollen's reagent is used for the detection of aldehyde. When a solutio...
Text Solution
|
- Tollen's reagent is used for the detection of aldehyde. When a solutio...
Text Solution
|
- Tollen's reagent is used for the detection of aldehyde. When a solutio...
Text Solution
|
- Tollen's reagent is used for the detection of aldehyde. When a solutio...
Text Solution
|
- Chemical reaction involve interaction of atoms and molecules. A large ...
Text Solution
|
- Chemical reaction involve interaction of atoms and molecules. A large ...
Text Solution
|
- Chemical reaction involve interaction of atoms and molecules. A large ...
Text Solution
|
- Redox reactions play a pivotal role in chemistry and biology. The valu...
Text Solution
|
- Redox reactions play a pivotal role in chemistry and biology. The valu...
Text Solution
|
- Redox reactins play a pivoted role in chemistry and biology. The value...
Text Solution
|
- For the reduction of NO(3)^(-) ion in an aqueous solution E^(@) is +0....
Text Solution
|
- The concentration of potassium ions inside a biological cell is at lea...
Text Solution
|
- The concentration of potassium ions inside a biological cell is at lea...
Text Solution
|