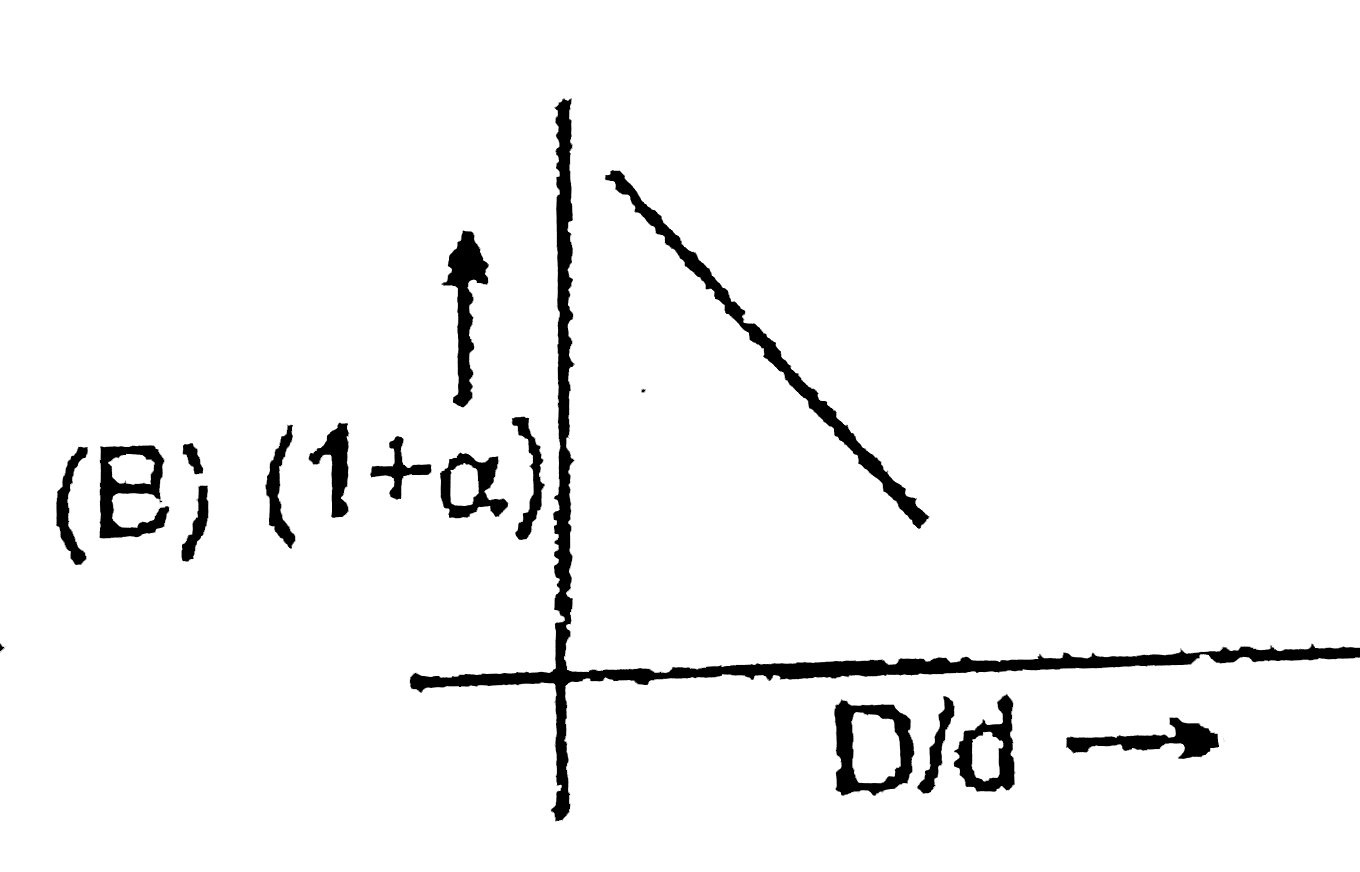
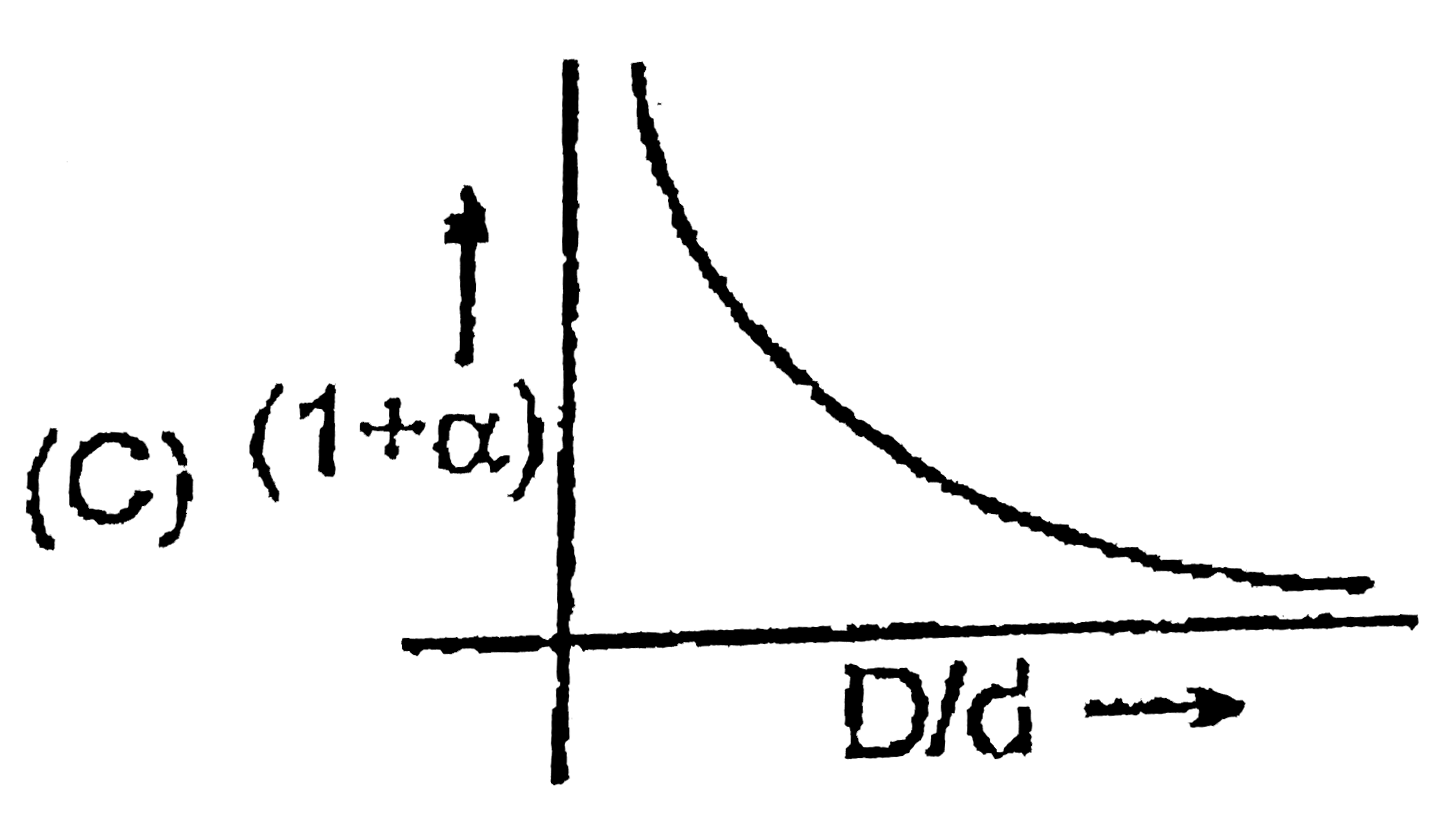
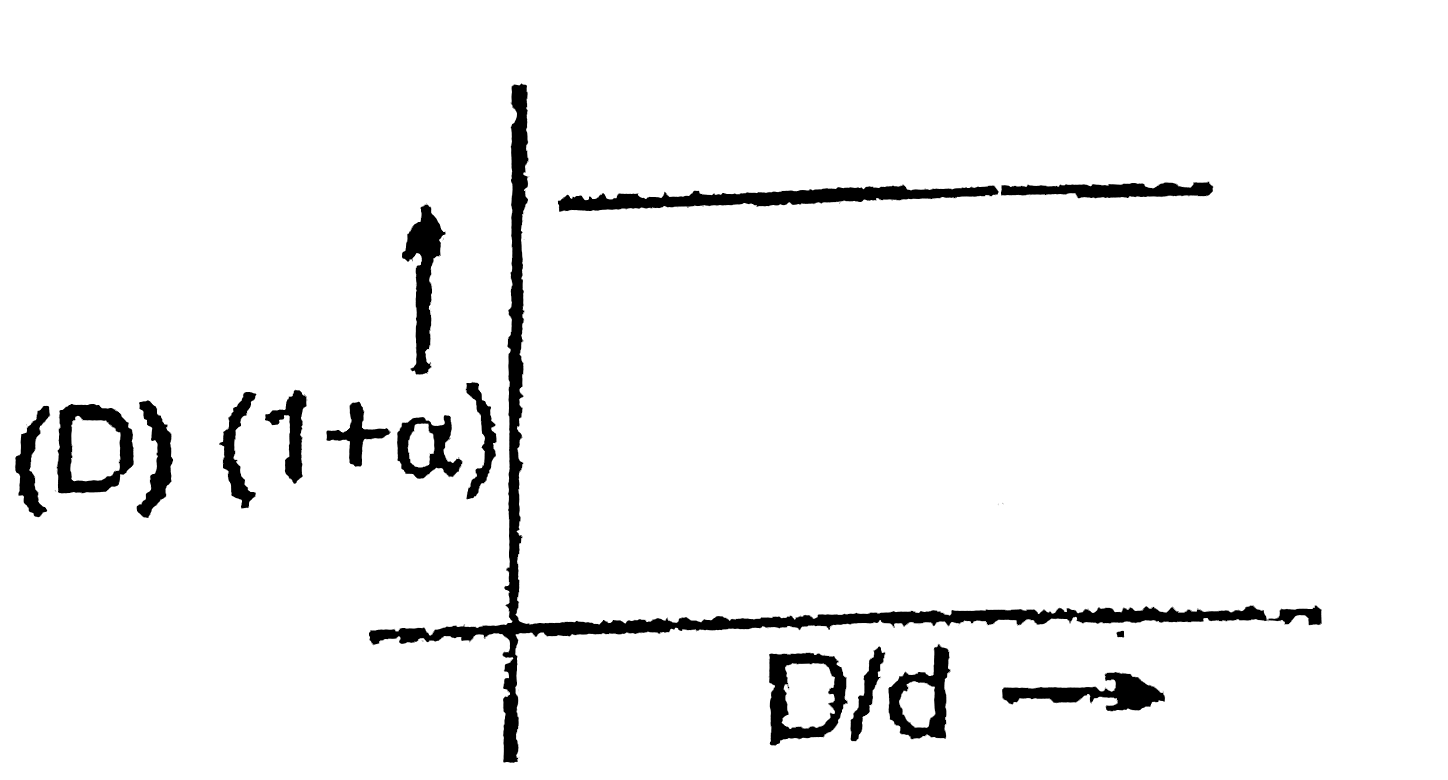
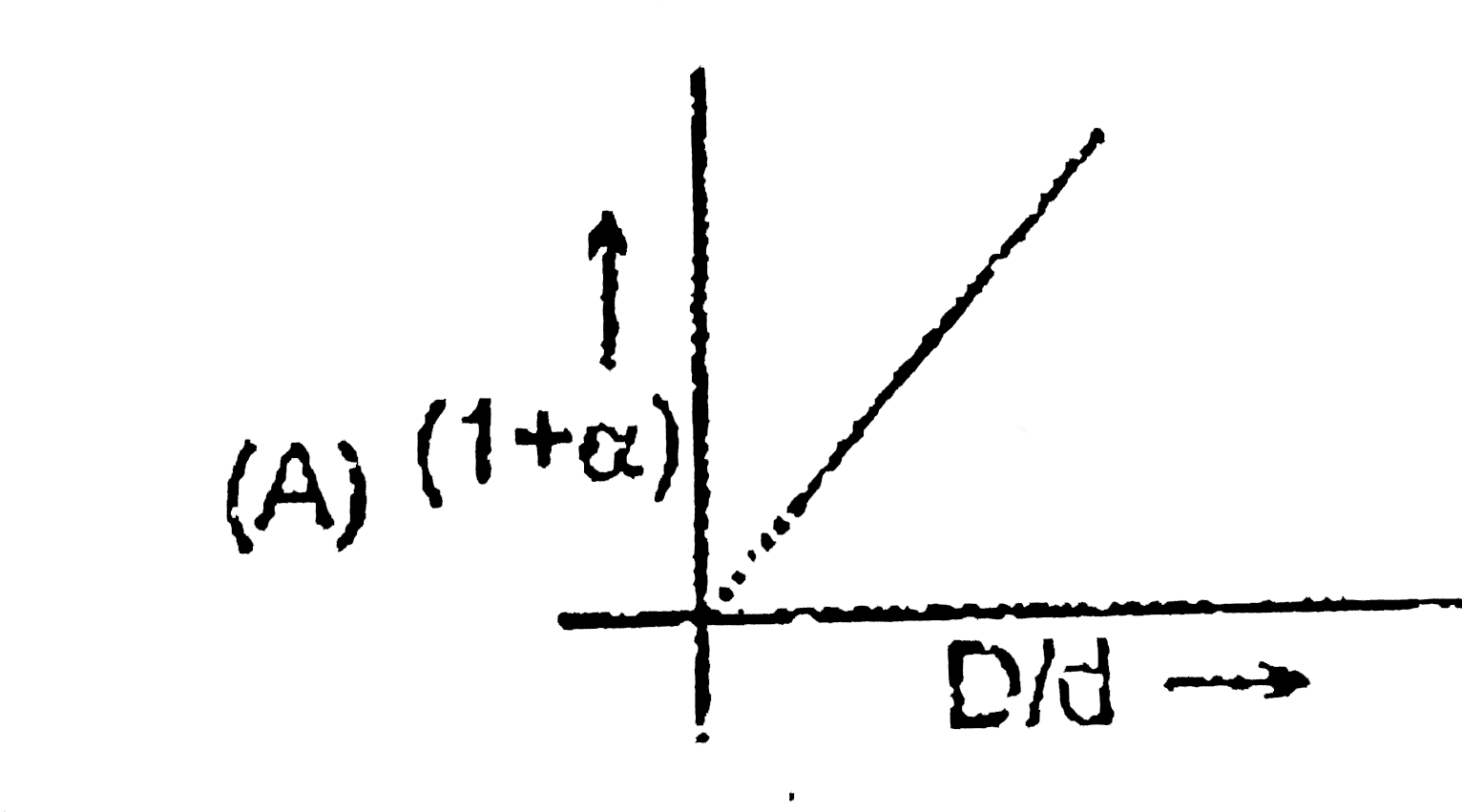A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VIBRANT-REVIEW TEST-PART - III : CHEMISTRY
- For the dissociation of NH(3)(g) as 2NH(3)(g)iffN(2)(g)+3H(2)(g), the...
Text Solution
|
- The gaseous reaction 2A(g) + nB(g) iff mC(g) is represented by foll...
Text Solution
|
- According to Slater's Rule effective nuclear charge of Na is x and of ...
Text Solution
|
- H^(+) ions are in excited state from where maximum 6 types of photons ...
Text Solution
|
- x = number of structure of molecular formula C(4)H(10)O which give fum...
Text Solution
|
- Determine the value of ((x+y^(2)))/(2) for Cr^(3+) ion Where x = nu...
Text Solution
|
- An alpha-particle having kinetic energy x MeV falls on a Cu-foil. The ...
Text Solution
|
- Identify the number of carbon atoms where oplus charge is delocalized.
Text Solution
|
- Electrons in a sample of H-atoms make transition from state n = y to s...
Text Solution
|
- Choose the incorrect statement(s) :
Text Solution
|
- In which of the following aromatic product is obtained
Text Solution
|
- I^(-) or the reaction I(2) (g) iff 2I(g). K(c )=6.25xx10^(-6) at 90...
Text Solution
|
- Phenol is less acidic than
Text Solution
|
- Give the correct order of initials T(True) for following statements :
Text Solution
|
- For similar nature of below graph where n le 5, select correct stateme...
Text Solution
|
- The electronic configuration of four element's are : (I) [Kr] 5s^(1...
Text Solution
|
- The rate consant for the forward reation A(g) iff 2B(g) is 1.5 xx 1...
Text Solution
|
- The value of four quantum number for the last e^(-) of atom of element...
Text Solution
|
- The value of four quantum number for the last e^(-) of atom of element...
Text Solution
|
- Acid are those compounds which give proton and base are those compound...
Text Solution
|
- Acid are those compounds which give proton and base are those compound...
Text Solution
|