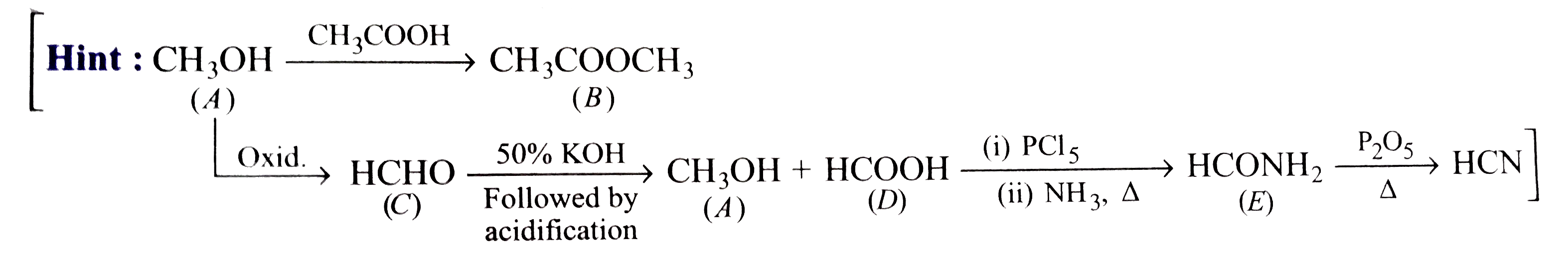
Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Brain storming problems|30 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Objective questions|498 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise problem for practise|31 VideosBASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|4 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CARBOXYLIC ACIDS AND THEIR DERIVATIVES-Problem based on structure and properties
- Compound (A) C(5)H(5)O(2) liberated carbon dioxide on reaction with so...
Text Solution
|
- An acidic compound (A) C(4)H(8)O(3) loses its optically activity on st...
Text Solution
|
- An acid [A] C(8)H(7)O(2)Br on bromination in the presence of FeBr(3) g...
Text Solution
|
- An organic compound (A),C(8)H(4)O(3) in dry benzene in the presence of...
Text Solution
|
- Show a sequence of reactions b y which the ester propyl propionate, CH...
Text Solution
|
- A liquid (X) having a molecular formula C(6) H(12) O(2) is hydrolysed ...
Text Solution
|
- An organic compound (A) on treatment with acetic acid in the presence ...
Text Solution
|
- The molecular formula of an organic compound is C(3)H(6)O(2). This com...
Text Solution
|
- An organic compound (A) on treatment with ethyl alcohol gives a carbox...
Text Solution
|
- One mole of an organic amide (A) on alkaline hydrolysis gives one mole...
Text Solution
|
- Compound (A) (C(6)H(12)O(2)) on reduction with LiAlH(4) yielded two co...
Text Solution
|
- In the following reactions. Identify the compounds A, B, C and D PCl(...
Text Solution
|
- Two moles of an ester (A) are condensed in the presence of sodium etho...
Text Solution
|
- An Organic compound (A) C(4)H(7)Cl(3) yields (B) with KOH(aq.) (B) on ...
Text Solution
|
- In the following reactions, identify the compounds (A), (B), (C) and R...
Text Solution
|
- A pleasant smelling optically active ester (A) has M.W. = 186. It does...
Text Solution
|
- C(5)H(10) (A) can show Cannizzaro's reaction. (A) on treatment with Al...
Text Solution
|
- An organic compound (A) reacts with ethanol to give (B) and (C). On ac...
Text Solution
|
- Compound (A), C(5)H(11)NO is not soluble in cold dilute alkaline or ac...
Text Solution
|
- An organic dibasic acid (A) gave the following on analysis: C = 57.8%,...
Text Solution
|