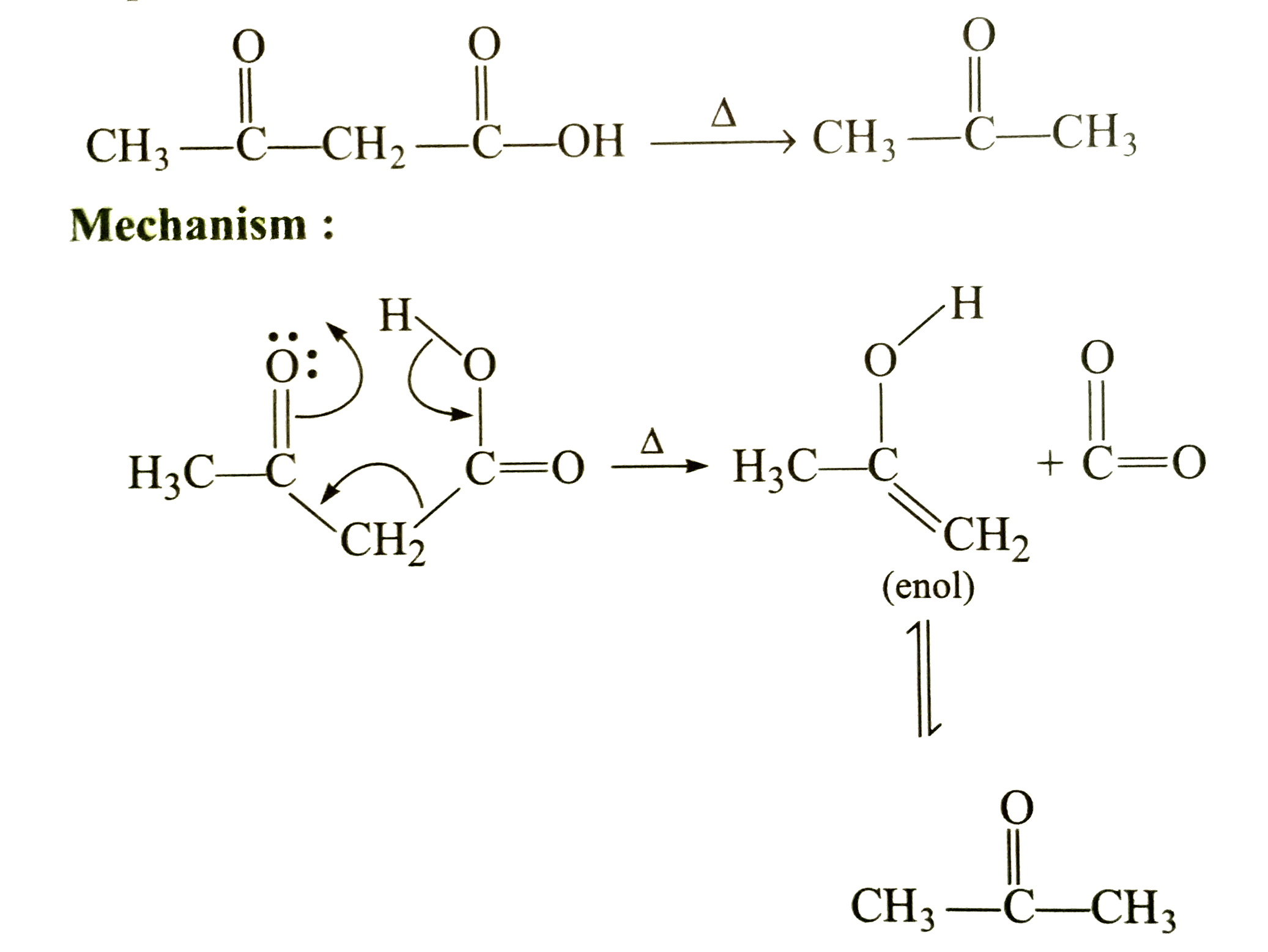
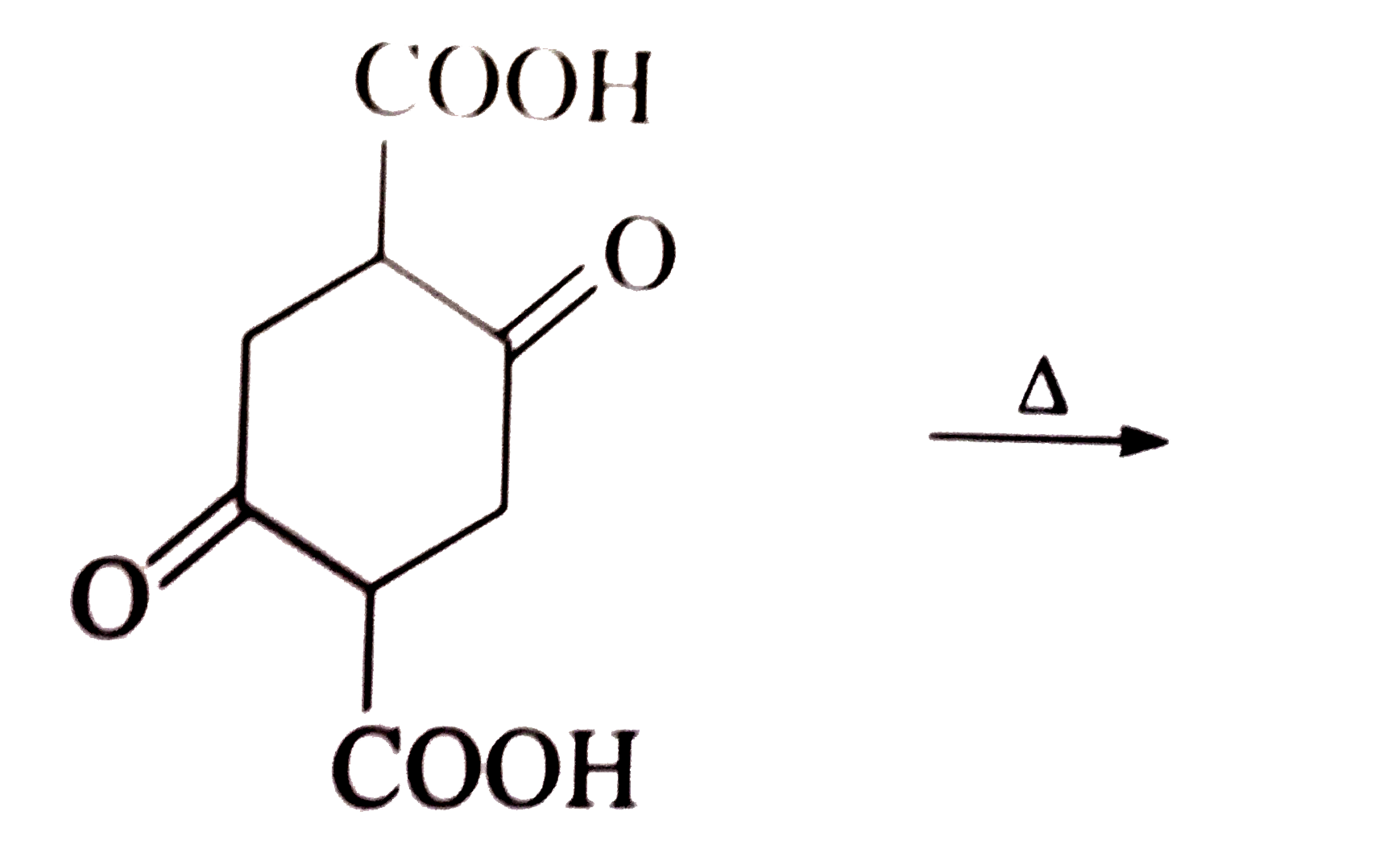
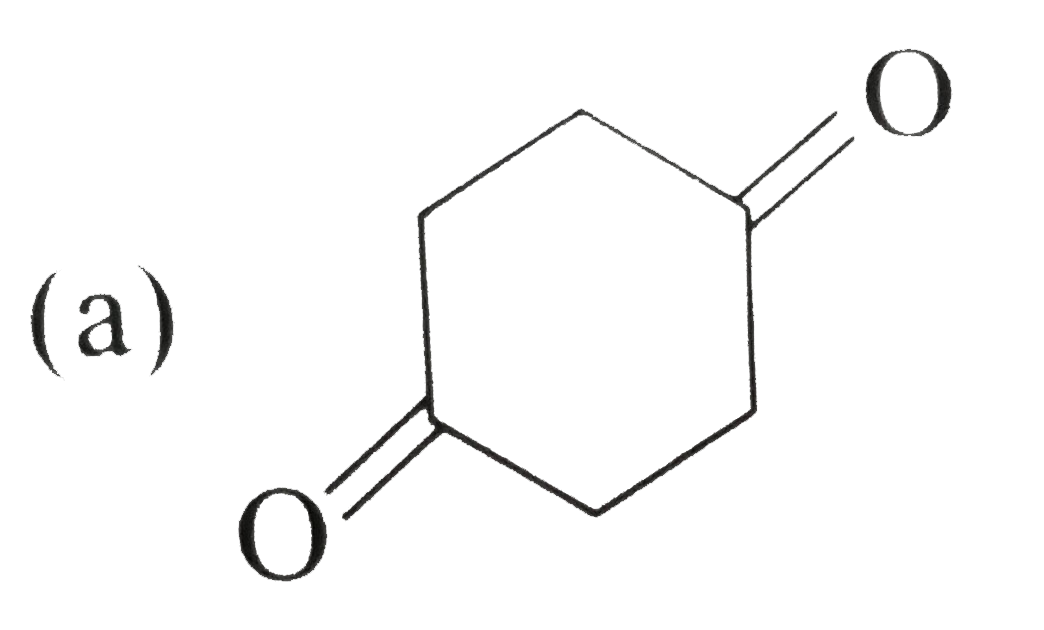
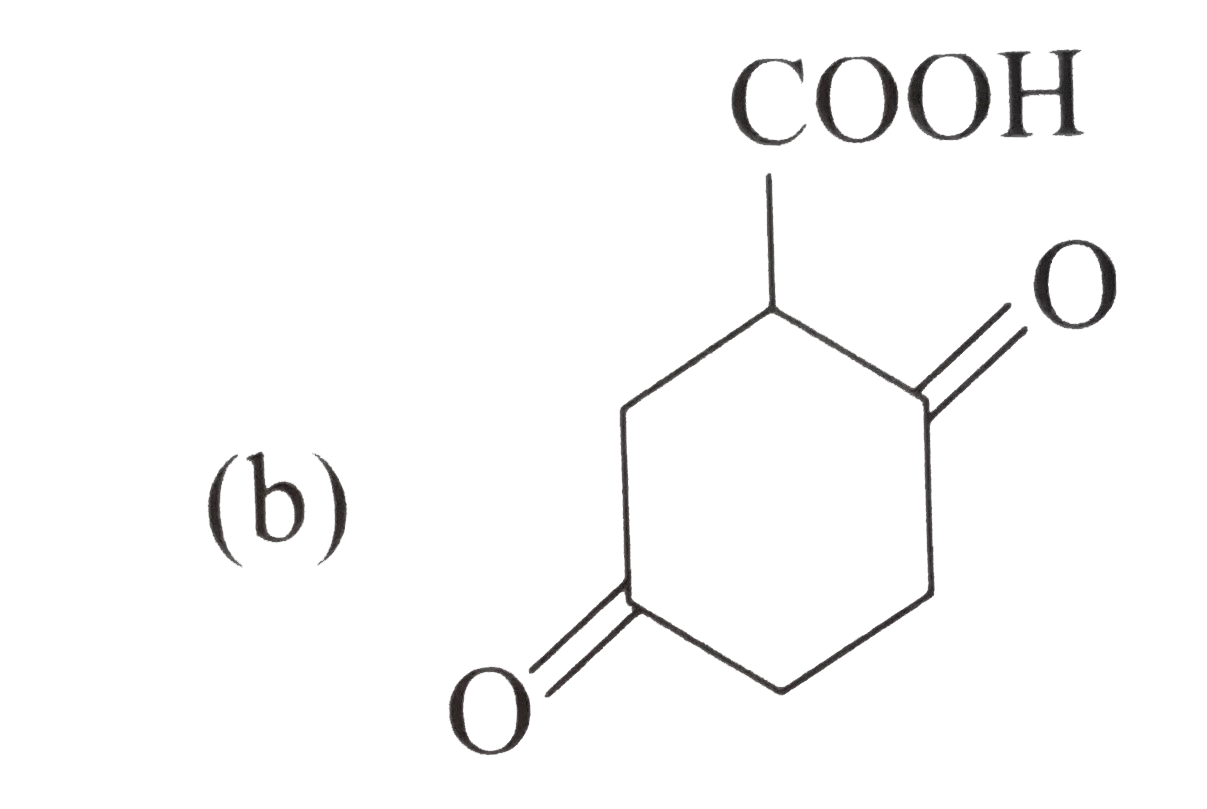
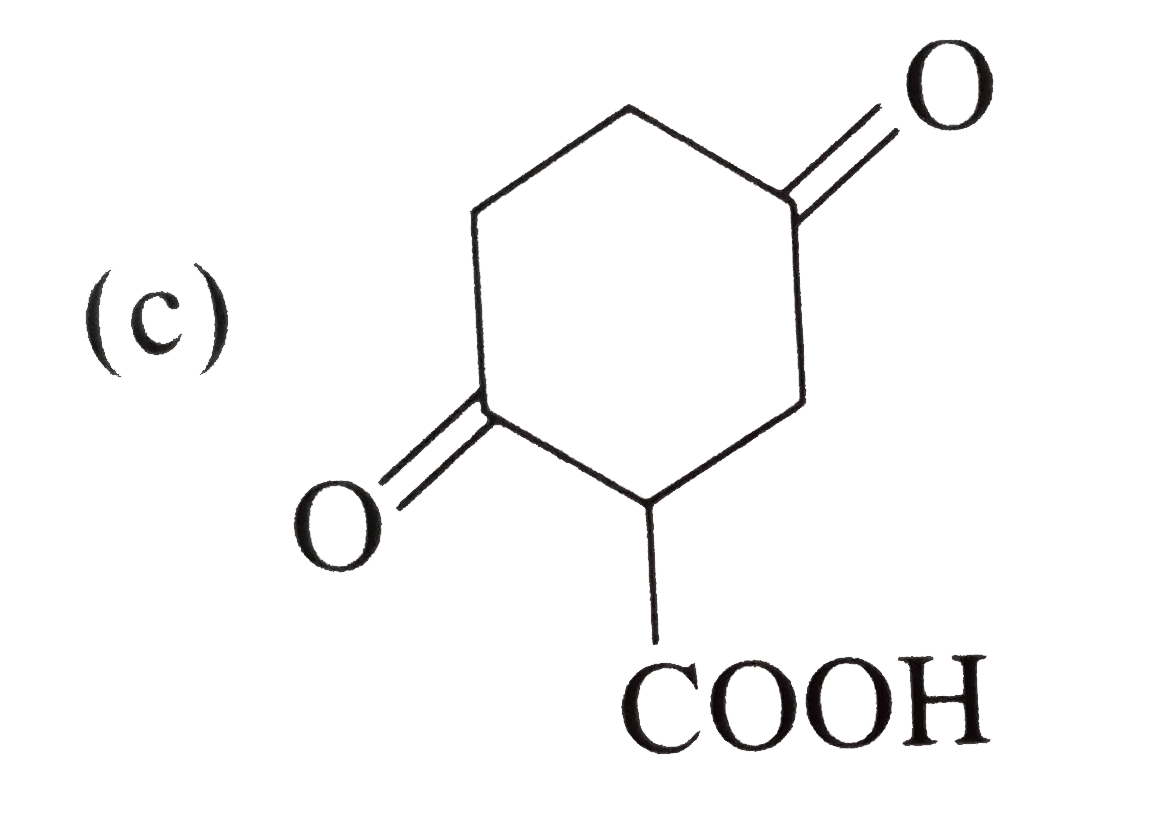
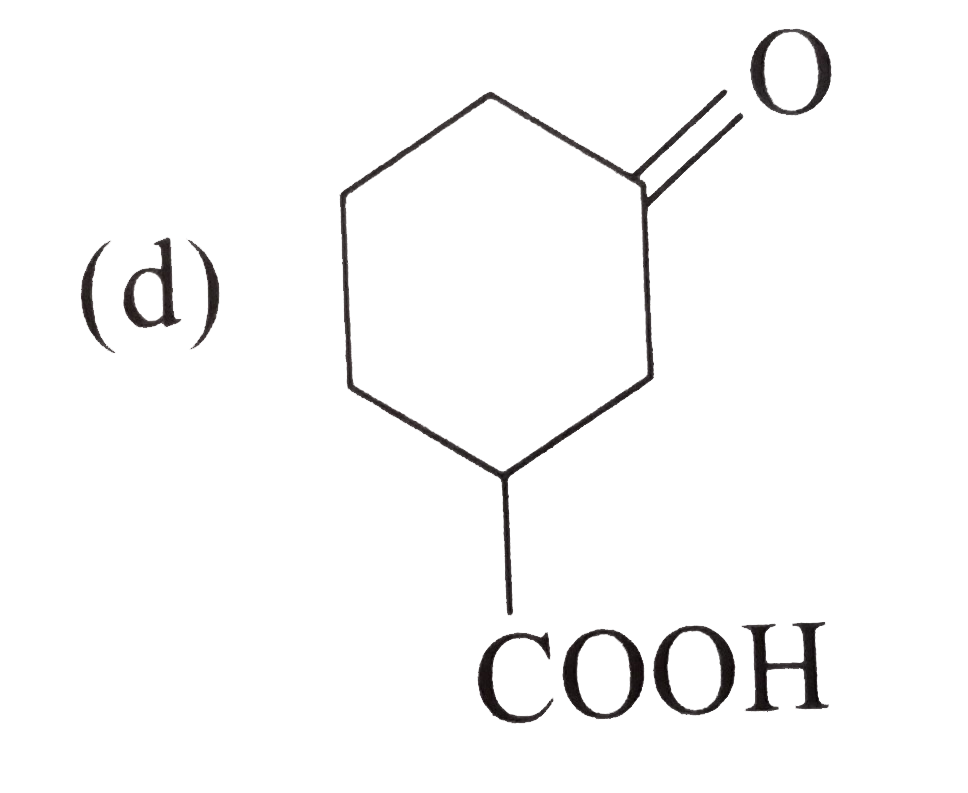A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Brain storming problems|30 VideosBASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|4 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CARBOXYLIC ACIDS AND THEIR DERIVATIVES-Objective questions
- P and Q are isomers of dicarboxyhc acid C(4)H4O(4) .Both decolourize B...
Text Solution
|
- In the following reaction sequences V and W are respectively
Text Solution
|
- The decarboxylation of beta-ketoacids beta,gamma-unsaturated acid an g...
Text Solution
|
- The decarboxylation of beta-ketoacids beta,gamma-unsaturated acid an g...
Text Solution
|
- The decarboxylation of beta-ketoacids beta,gamma-unsaturated acid an g...
Text Solution
|
- How many of the following compounds are soluble in sodium bicarbonate?...
Text Solution
|
- Cyclohexane carboxylic acid is treated with Cl(2) in phosphorus where ...
Text Solution
|
- The total number of carboxylic acid groups in the product P is :
Text Solution
|
- How many moles of CO(2) will releasd when following compound is heated...
Text Solution
|
- Identify the number of reagents that can be used for the following con...
Text Solution
|
- Examine the structure of following compounds and find out the number o...
Text Solution
|
- Among the following, the total number of compound soluble in aqueous N...
Text Solution
|
- Eaxmine the structural formula shown below and find out how many com...
Text Solution
|
- Match the following
Text Solution
|
- Match the following
Text Solution
|
- This reaction takes place between two molecules of esters (same or dif...
Text Solution
|
- This reaction takes place between two molecules of esters (same or dif...
Text Solution
|
- This reaction takes place between two molecules of esters (same or dif...
Text Solution
|
- This reaction takes place between two molecules of esters (same or dif...
Text Solution
|
- This reaction takes place between two molecules of esters (same or dif...
Text Solution
|