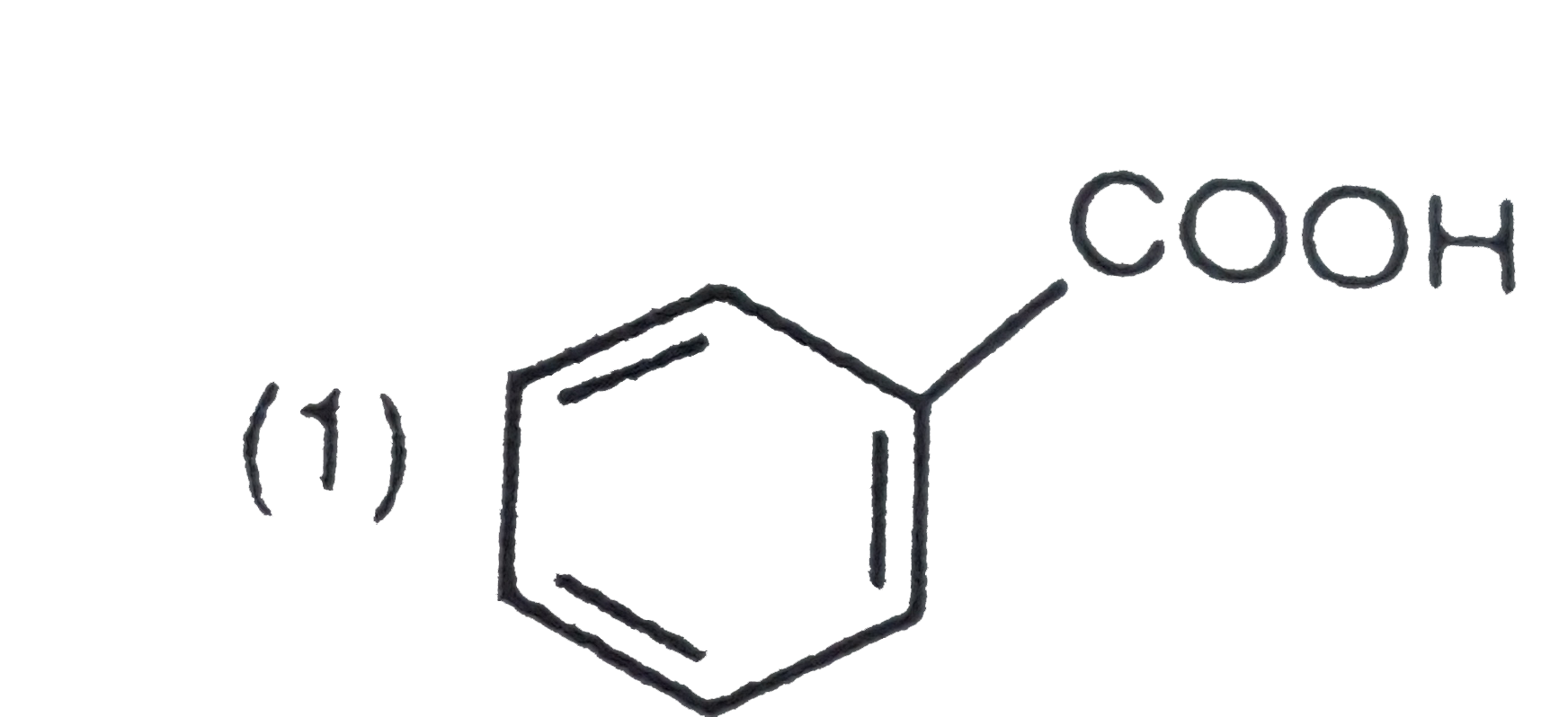
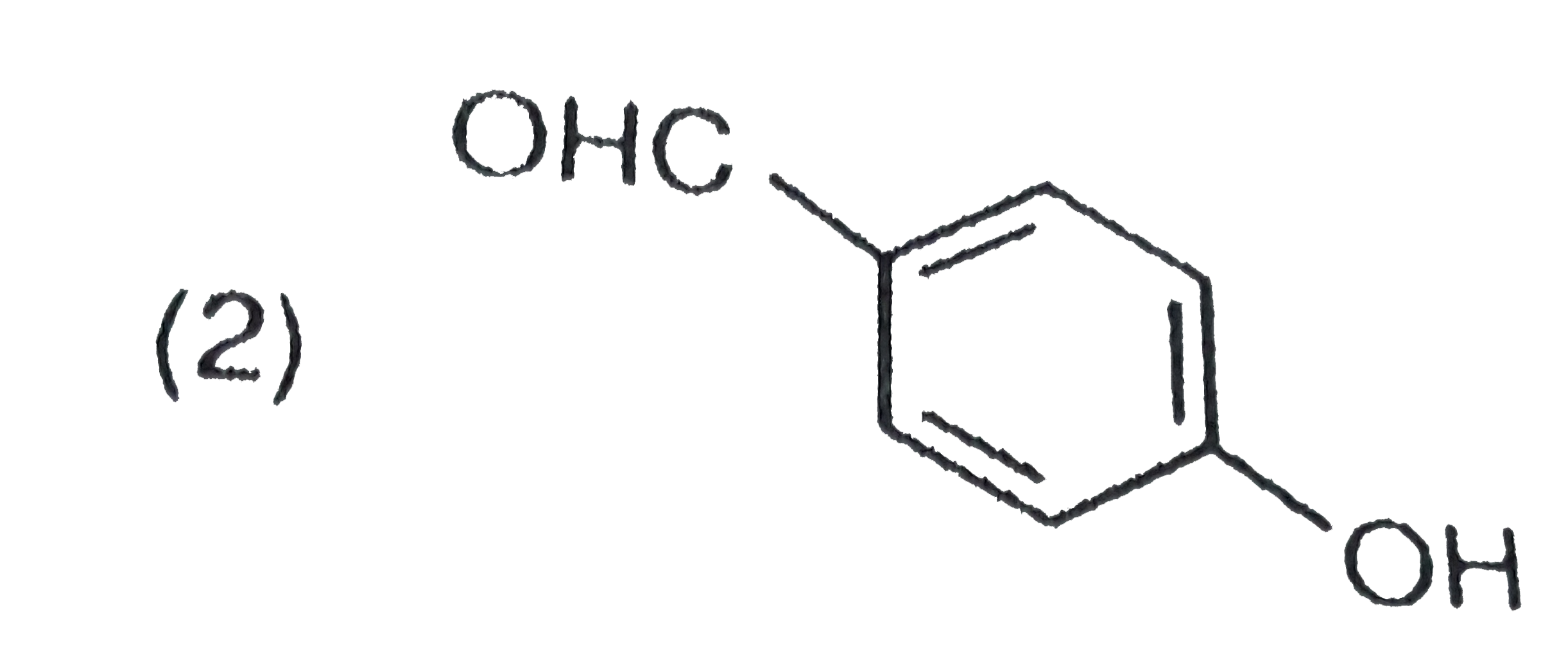
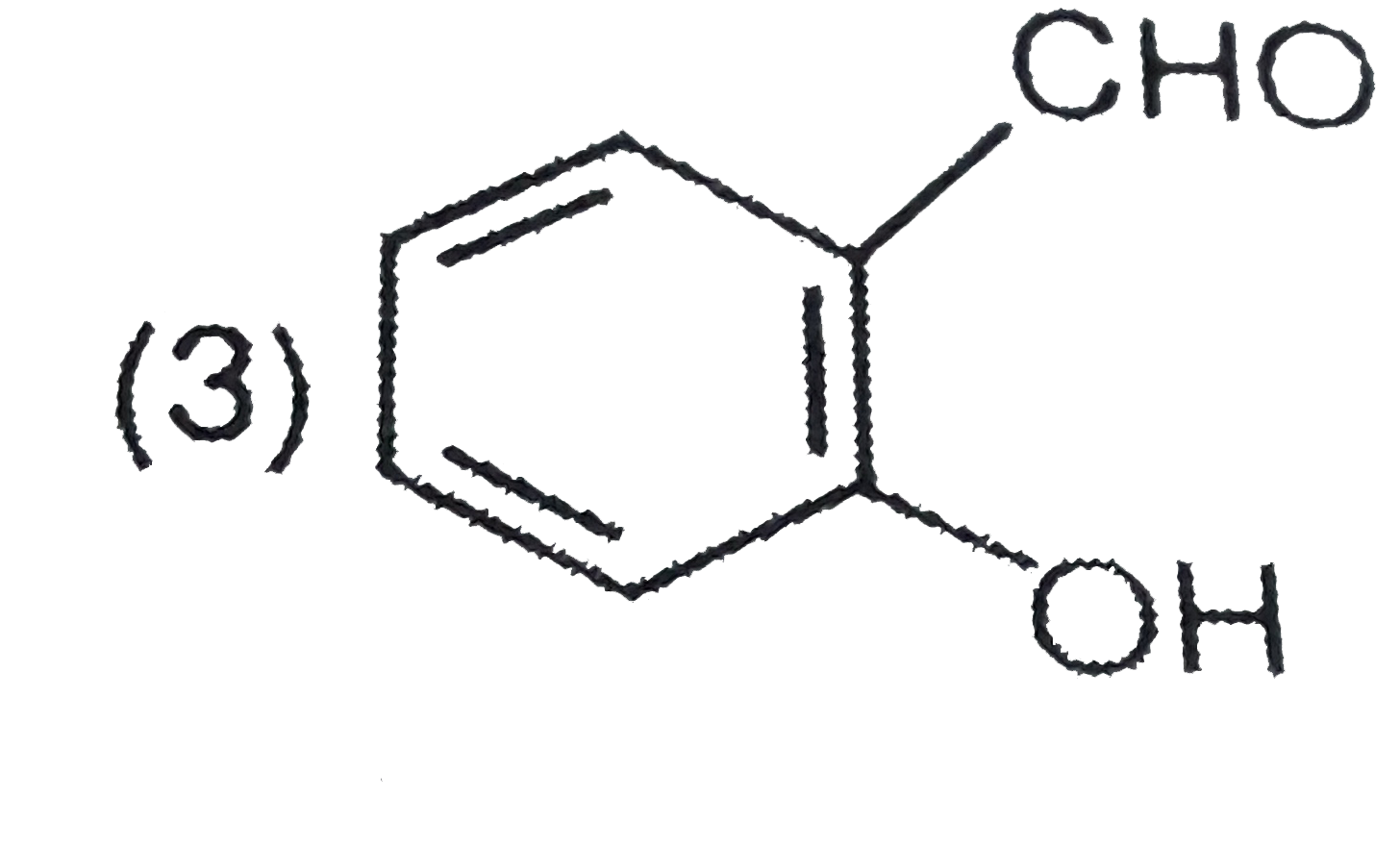
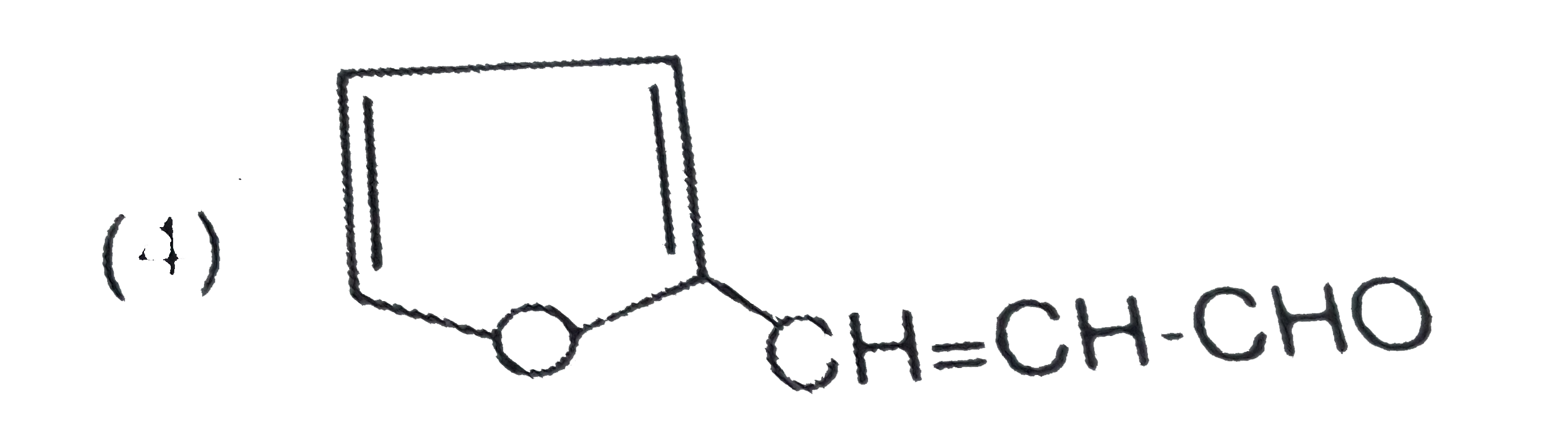
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE|Exercise PART-I PRACTICE TEST-1(IIT-JEE MAIN PATTERN)|30 VideosAROMATIC COMPOUNDS
RESONANCE|Exercise PART-II: NATIONAL STANDARD EXAMINATION IN CHEMISTRY (NSEC) STAGE-I|31 VideosAROMATIC COMPOUNDS
RESONANCE|Exercise PART-II JEE MAIN|19 VideosATOMIC STRUCTURE
RESONANCE|Exercise PHYSICAL CHEMISTRY(ATOMIC EQUILIBRIUM)|48 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-AROMATIC COMPOUNDS -JEE(MAIN) ONLINE PROBLEM
- The following reaction is known as :
Text Solution
|
- Complete reduction of benzene-diazonium chloride with Zn/HCl gives :
Text Solution
|
- Conversion of benzene diazonium chloride to chloro benzene is an examp...
Text Solution
|
- In a set of reactions p-nitrotulene yielded a product E. The product E...
Text Solution
|
- Fluorination of an aromatic ring is easily accompolished by treating a...
Text Solution
|
- The major product of the following reaction is :
Text Solution
|
- The increasing order of diazotization of the following compound is:
Text Solution
|
- Products A and B formed in the following reactions are respectively:
Text Solution
|
- The major product of the following reaction is:
Text Solution
|
- The major product of the following reaction:
Text Solution
|
- The products formed in the reaction of cumene with O(2) followed by tr...
Text Solution
|
- The major product formed in the reaction given below will be:
Text Solution
|
- An aromatic compound 'A' having molecular formula C(7)H(6)O(2) on trea...
Text Solution
|
- The major product of the following reaction:
Text Solution
|
- A compound 'X' on treatment with Br(2)/NaOH, provided C(3)H(9)N, which...
Text Solution
|
- The major product of the following reaction is:
Text Solution
|