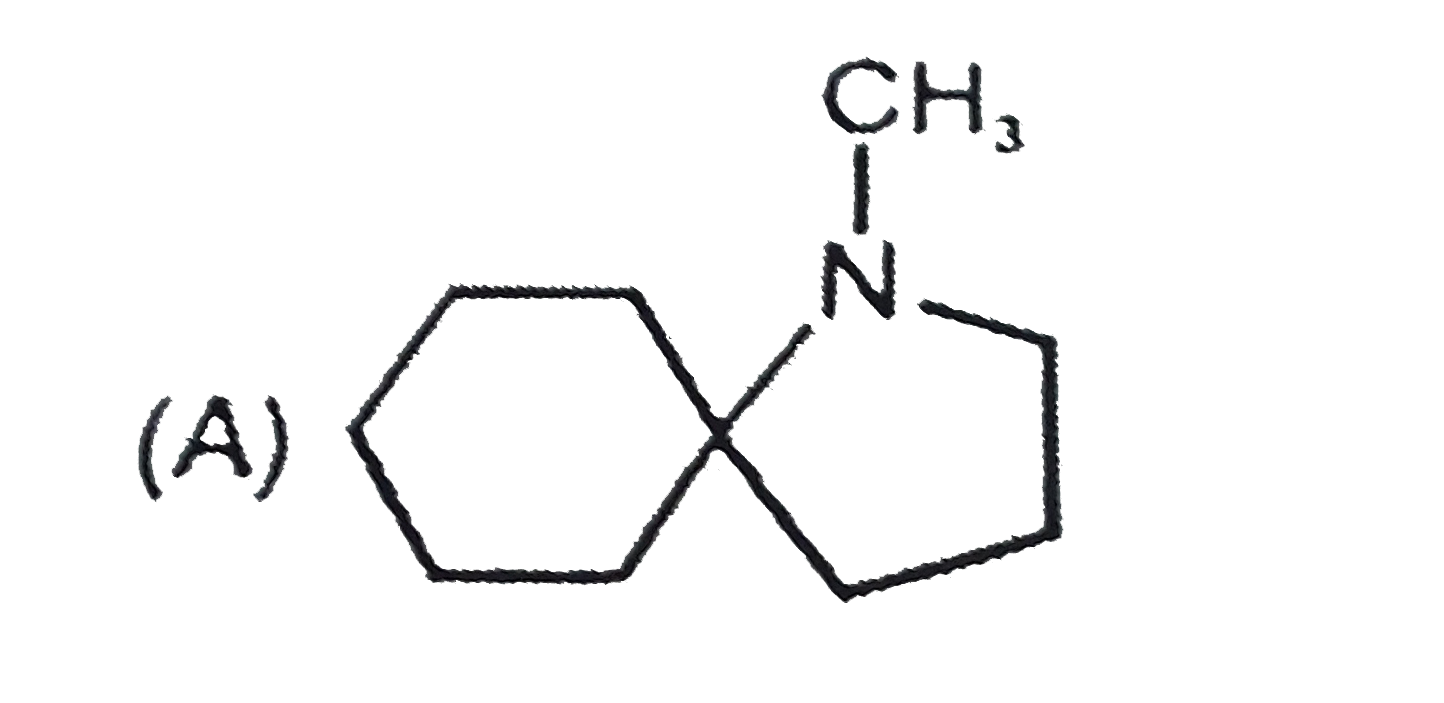
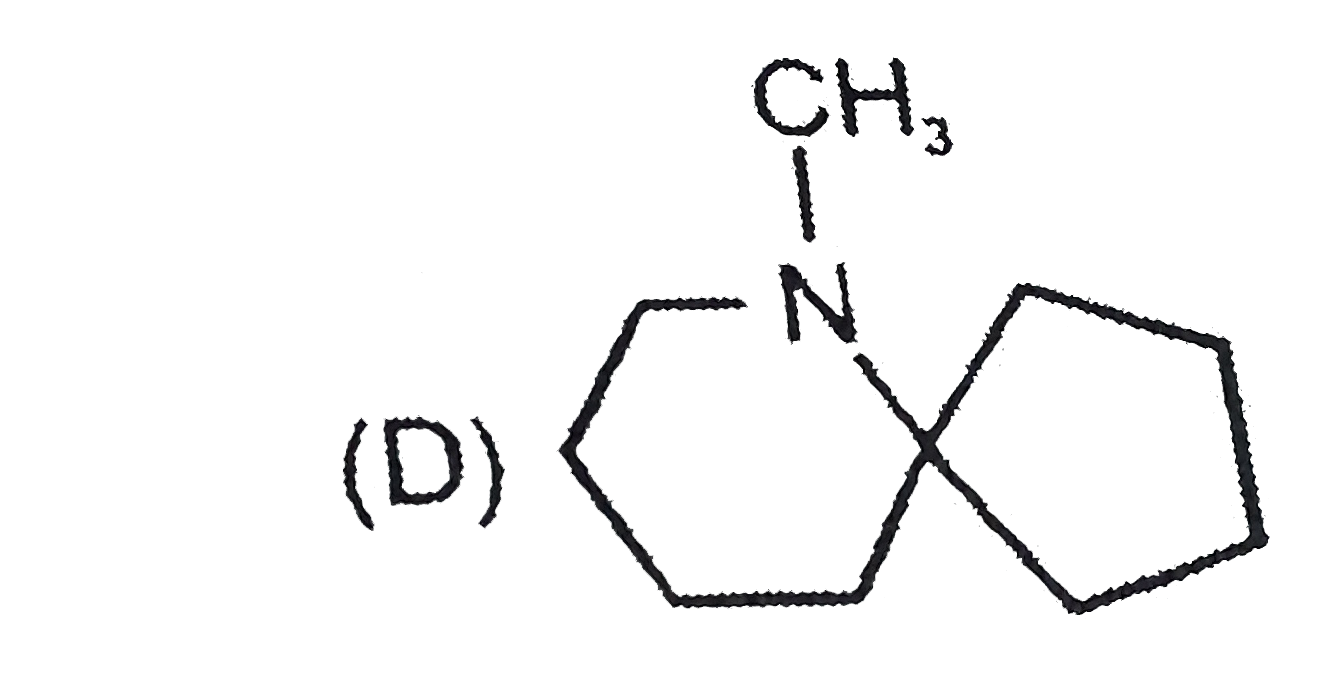A
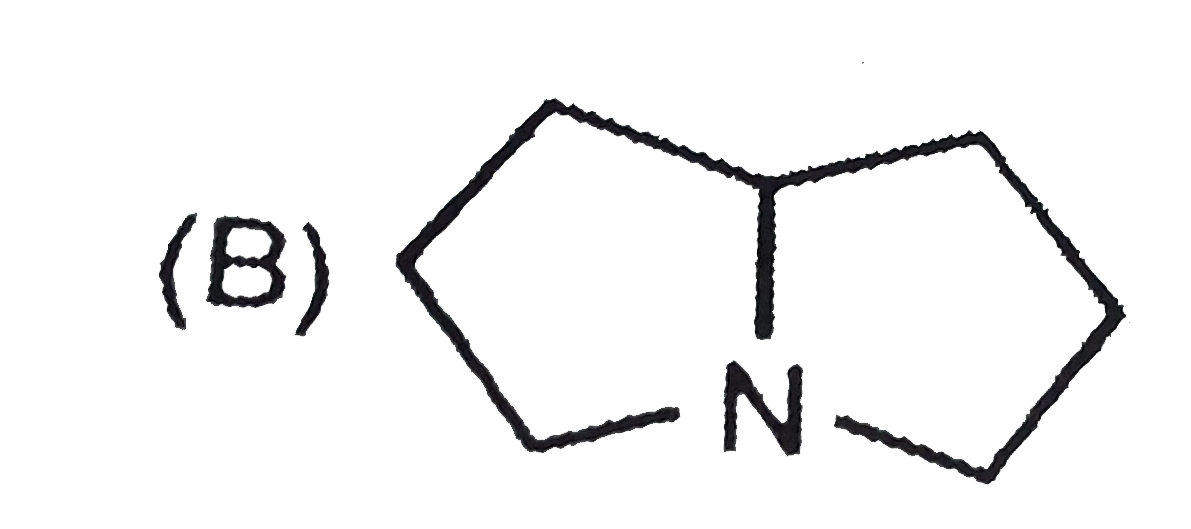
B
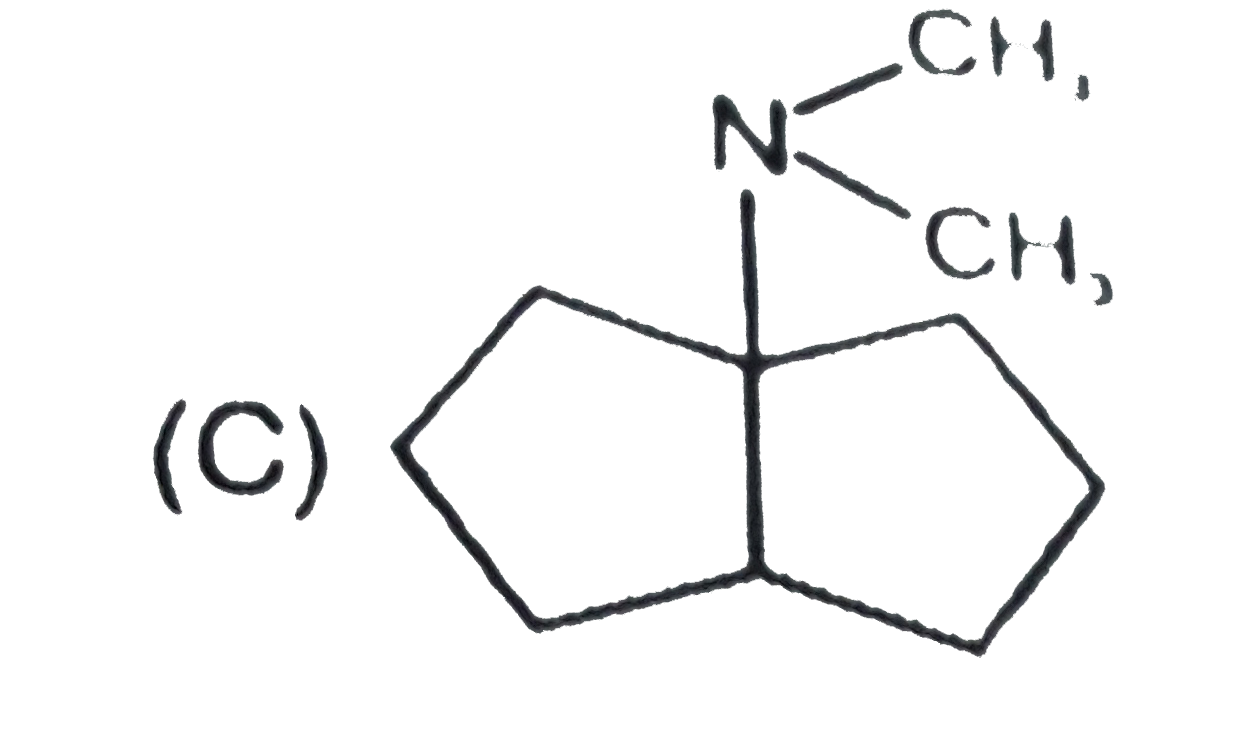
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE|Exercise PART-III: PRACTICE TEST-2(IIT-JEE(ADVANCED pattern))|7 VideosAROMATIC COMPOUNDS
RESONANCE|Exercise Section -2 (one or more than one options correct type)|7 VideosAROMATIC COMPOUNDS
RESONANCE|Exercise PART-I PRACTICE TEST-1(IIT-JEE MAIN PATTERN)|30 VideosATOMIC STRUCTURE
RESONANCE|Exercise PHYSICAL CHEMISTRY(ATOMIC EQUILIBRIUM)|48 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-AROMATIC COMPOUNDS -PART-II: NATIONAL STANDARD EXAMINATION IN CHEMISTRY (NSEC) STAGE-I
- The product obtained when 4-hydroxybenzene sulphonic acid is treated ...
Text Solution
|
- The most appropriate reaction for the conversion of bromobenzene to be...
Text Solution
|
- p-bromoaniline is prepared from aniline via
Text Solution
|
- Which one of the following has the highest melting point ?
Text Solution
|
- On bromination, the electron rich phenoxide ion will be attacked most ...
Text Solution
|
- The sequence of decreasing aromaticity in the above compounds is
Text Solution
|
- Can the amino group, in the aniline molecule, become meta-directing in...
Text Solution
|
- Consider the following reactions the major product (Y) of the re...
Text Solution
|
- The product of Reimer-Tiemann reaction is a
Text Solution
|
- The nitrogen atom in the following cyclic compounds can be removed as ...
Text Solution
|
- X underset("ether")overset(Mg)rarrY underset(H^(+))overset("Dry " CO(2...
Text Solution
|
- Salicylic acid on treatment with bromine water will give
Text Solution
|
- The product P obtained through the following sequence of reactions is
Text Solution
|
- Triethylamine is reacted with a peracid to obtain. The nitrogen atom i...
Text Solution
|
- An organic base (X) reacts with nitrous at 0^(@) to give a clear solut...
Text Solution
|
- organic compound sometimes adjust their electronic as well as steric s...
Text Solution
|
- The major product 'S' of the following reaction sequence is:
Text Solution
|
- The product of the following reaction is
Text Solution
|
- The major product of the following reaction is:
Text Solution
|
- The major product 'X' formed in the following reaction is
Text Solution
|