Text Solution
Verified by Experts
Topper's Solved these Questions
D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE|Exercise Additional Problems for Self practice (APSP)|30 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE|Exercise National Standard Examination In chemistry|35 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE|Exercise Exercise 2|40 VideosCHEMICAL EQUILIBRIUM
RESONANCE|Exercise Advanced Level Problems (Part-3)(Stage-5)|2 VideosGASEOUS STATE
RESONANCE|Exercise PHYSICAL CHMISTRY (Gaseous State)|47 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS -Exercise 3
- The pair of compounds having metals in their highest oxidation state i...
Text Solution
|
- Which of the following pair of compounds is expected to exhibit same c...
Text Solution
|
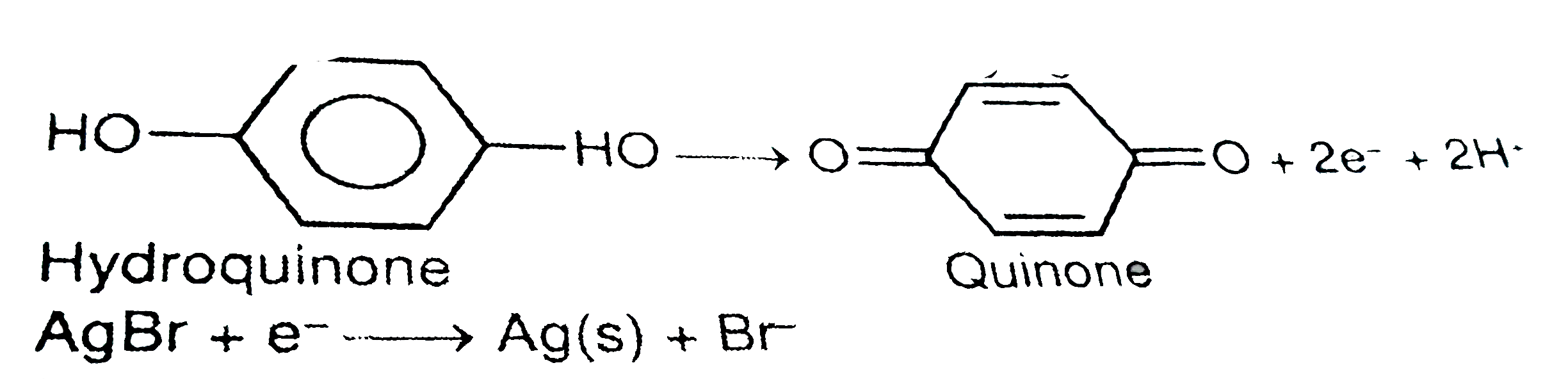
- Give equation and describe the process for the developing of black and...
Text Solution
|
- Identify (A),(B) and (C). Also explain colour difference between MCl(4...
Text Solution
|
- Match the reaction in column I with nature of the reactions/type of th...
Text Solution
|
- Among the following , the coloured compound is
Text Solution
|
- Find the oxidation number of Mn in the product of alkaline oxidative f...
Text Solution
|
- Reduction of the metal centre in aqueous permanganate ion involves
Text Solution
|
- The colour of light absorbed by an aqueous solution of CuSO(4) is
Text Solution
|
- Which of the following halides react(s) with AgNO(3(aq)) to give a pre...
Text Solution
|
- Consider the following list of reagent "Acidified" K(2)Cr(2)O(7), "a...
Text Solution
|
- Which of the following statement are correct about Cr^(2+) (Z = 24) an...
Text Solution
|
- Fe^(3+) is reduced to Fe^(3+) by sing
Text Solution
|
- In the following sequence in aqueous solution, the species X, Y and Z,...
Text Solution
|
- Which of the following combination will produce H(2) gas ?
Text Solution
|
- Number of electrons transferred in each case when KMnO(4) acts as an o...
Text Solution
|
- which of the following ions has the maximum magnetic moment ?
Text Solution
|
- The most common oxidation states of cerium are
Text Solution
|
- What happen when a solution of potassium chromate is treated with an e...
Text Solution
|
- Which one of the following nitrates will leaves behind a metal on stro...
Text Solution
|