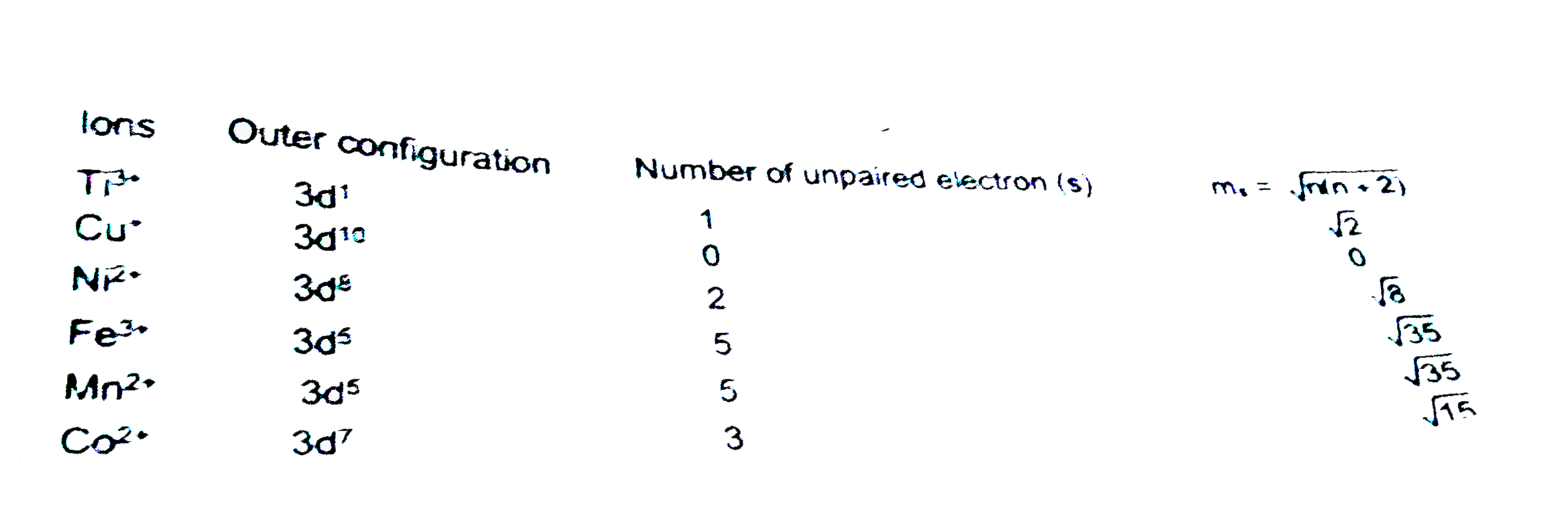Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE|Exercise Paragraph|3 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE|Exercise Match the column|1 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE|Exercise One or more than one options correct type|10 VideosCHEMICAL EQUILIBRIUM
RESONANCE|Exercise Advanced Level Problems (Part-3)(Stage-5)|2 VideosGASEOUS STATE
RESONANCE|Exercise PHYSICAL CHMISTRY (Gaseous State)|47 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS -One Integer Value Correct Type.
- How many of the followign are coloured or paramagnetic or coloured and...
Text Solution
|
- How many of the following ions have spin mangnetic moment more than 4...
Text Solution
|
- In how many of the following reactions, colored precipitate is obtaine...
Text Solution
|
- In the above reaction scheme, MnO(2) appears more than once. From (A)-...
Text Solution
|
- How many of the following compounds are diamagnetic and coloured? K(...
Text Solution
|
- How many of given statements are true for lanthanums? 1. The common ...
Text Solution
|