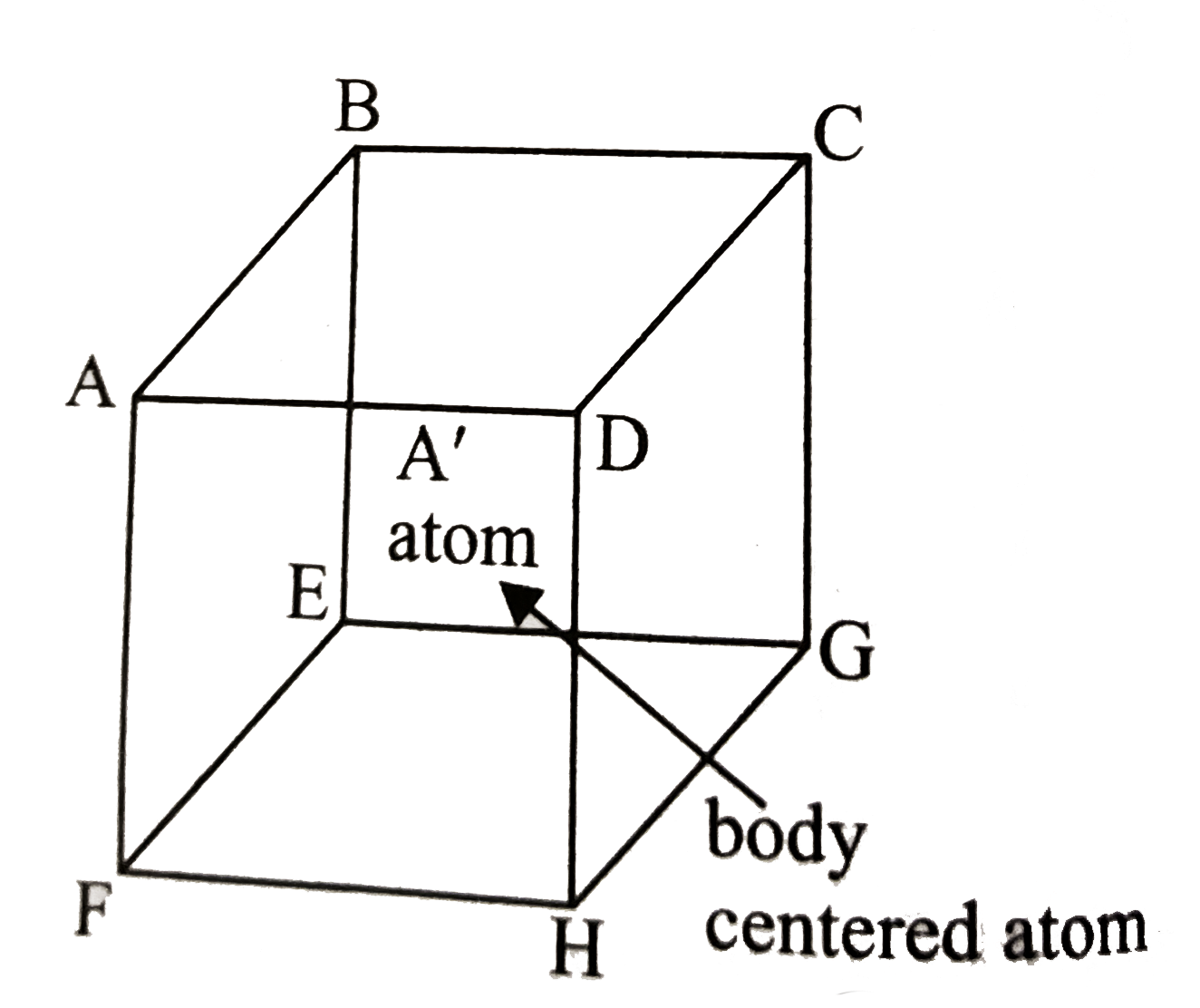
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
RESONANCE|Exercise Exercise -2 (Part-IV Comprehension)|10 VideosSOLID STATE
RESONANCE|Exercise Exercise -3 (Part-I)|21 VideosSOLID STATE
RESONANCE|Exercise Exercise -2 (Part-II Single correct double value integer type)|9 VideosS BLOCK ELEMENTS
RESONANCE|Exercise INORGANIC CHEMISTRY(s-Block Elements)|36 VideosSTRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY
RESONANCE|Exercise Section -B|18 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-SOLID STATE-Exercise -2 (Part-III)
- Which of the following statements is/are false
Text Solution
|
- Amorphous solids can also be callled ………….. .
Text Solution
|
- In body-centred cubic lattice given below, the three disntances AB, AC...
Text Solution
|
- A metal crystallises in bcc. Find % fraction of edge length not covere...
Text Solution
|
- Select the correct statement(s) about three-dimensional hcp system.
Text Solution
|
- Which of the following is/are ture about HCP and CCP lattice?
Text Solution
|
- In which of the following arrangements, Octahedral voids are forme...
Text Solution
|
- the number of tetrahedral voids per unit cell in NaCl crystal is ...
Text Solution
|
- Which of the following statements are correct
Text Solution
|
- Which of the following is/are correct ?
Text Solution
|
- A perfect crystal of silicon (fig) is doped with some elements a...
Text Solution
|
- Which of the following statements are true about semiconductors ?
Text Solution
|