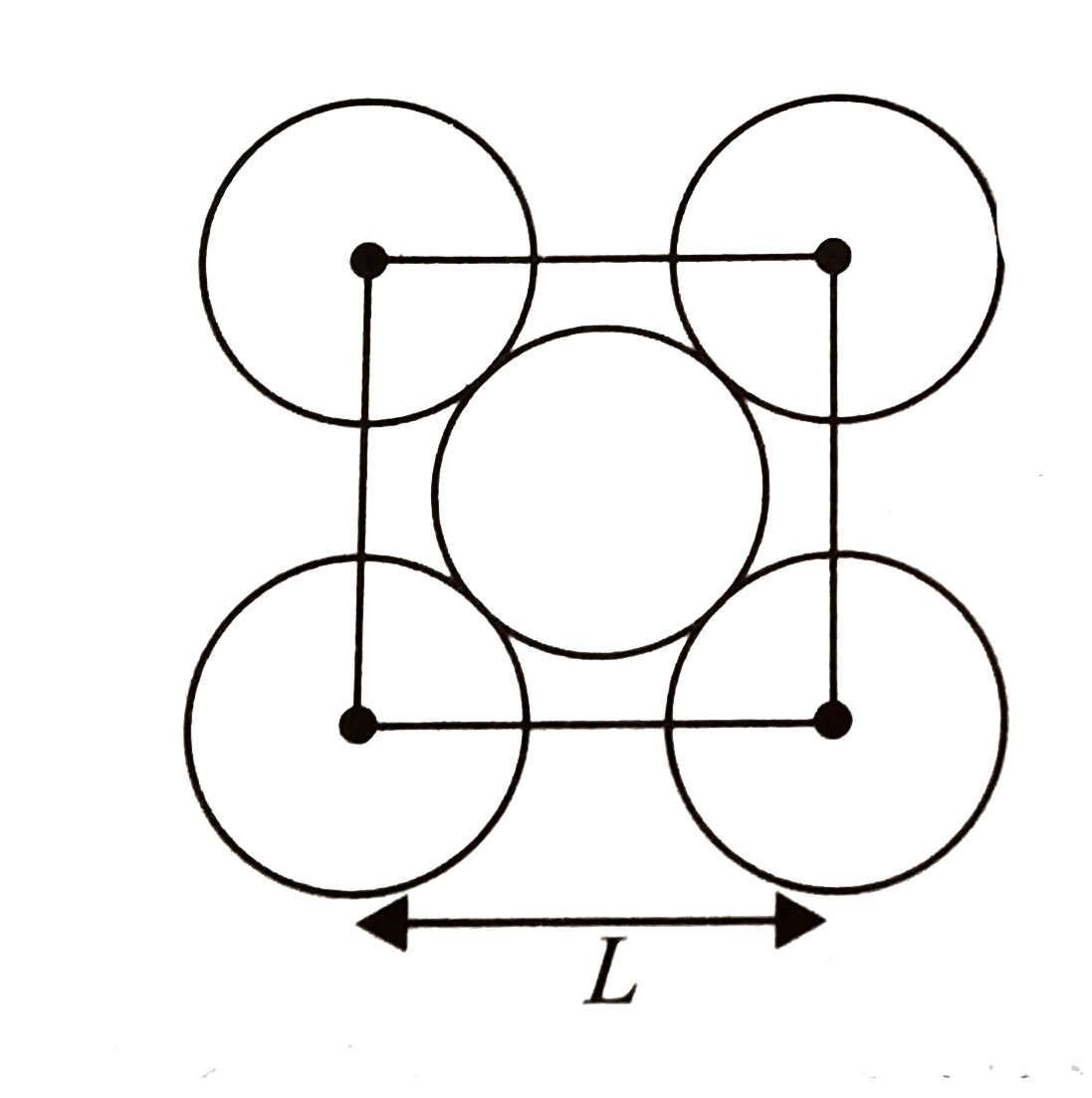A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
RESONANCE|Exercise Exercise -3 (Part-II)|18 VideosSOLID STATE
RESONANCE|Exercise Exercise -3 (JEE -MAIN)|12 VideosSOLID STATE
RESONANCE|Exercise Exercise -2 (Part-IV Comprehension)|10 VideosS BLOCK ELEMENTS
RESONANCE|Exercise INORGANIC CHEMISTRY(s-Block Elements)|36 VideosSTRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY
RESONANCE|Exercise Section -B|18 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-SOLID STATE-Exercise -3 (Part-I)
- In a solid AB having the NaCl structure, A atom occupies the corners o...
Text Solution
|
- A substance A(x)B(y) crystallizes in a face-centred cubic lattice in w...
Text Solution
|
- Marbles of diameter 10mm each to be arranged on a flat surface so that...
Text Solution
|
- A binary salt AB (formula weight = 6.023 Y amu, where Y is an arbitray...
Text Solution
|
- In which of the following crystals, alternate tetrahedral voids are oc...
Text Solution
|
- An element crystallizes in fcc lattice having edge length 400 pm Calcu...
Text Solution
|
- For a unit cell edge length = 5 Å, the element is of atomic mass 75, h...
Text Solution
|
- Match the crystal system/unit cells mentioned in Column I with their c...
Text Solution
|
- In hexagonal systems of crystals, a frequently ecounted arrangement of...
Text Solution
|
- In hexagonal systems of crystals, a frequently ecounted arrangement of...
Text Solution
|
- In a hexaonal system system of cycstals, a frequently encountered arra...
Text Solution
|
- The correct statement (s) regarding defects in solids is (are):
Text Solution
|
- The packing efficiency of a two-dimensional square unit cell shown bel...
Text Solution
|
- The number of hexagonal faces that are present in a truncated octahedr...
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- The arrangement of X^(ɵ) ions around A^(o+) ion in solid AX is given i...
Text Solution
|
- If the unit cell of a mineral has cubic close packed (ccp) array of ox...
Text Solution
|
- The correct statement (s) for cubic close packed (ccp) three dimension...
Text Solution
|
- A crystalline solid of a pure substance has a face-centred cubic struc...
Text Solution
|
- Consider an ionic solid MX with NaCl structure. Construct a new struct...
Text Solution
|