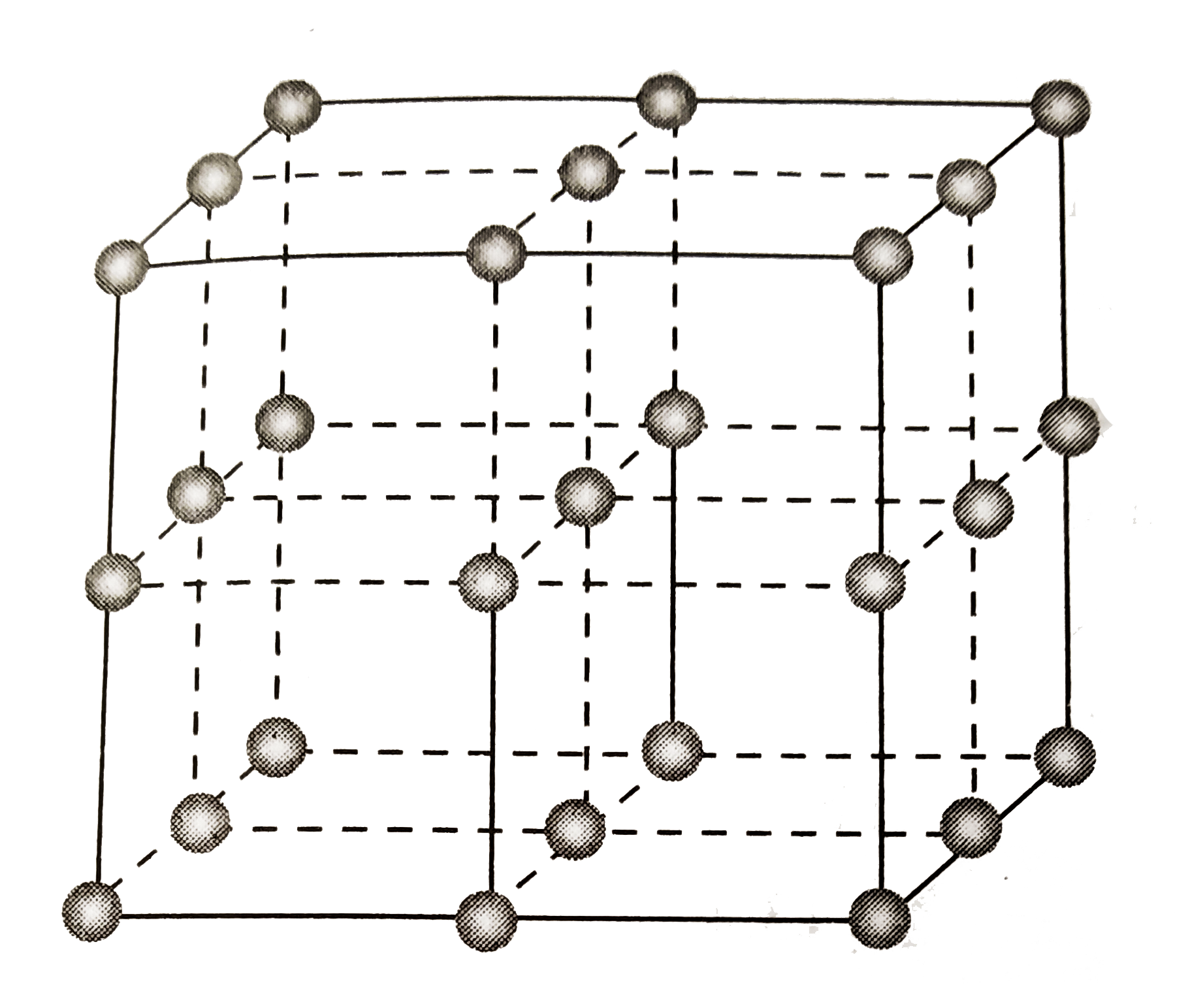
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-SOLID STATE-Part- IV
- In a face centered lattice of X and Y, X atoms are present at the corn...
Text Solution
|
- In a ccp structure of X atoms, Y atoms occupy all the octahedral holes...
Text Solution
|
- The following diagram shows the arrangement of lattice points with a =...
Text Solution
|
- A crystal is made of particles A and B . From fcc packing and B...
Text Solution
|
- Calculate the perimeter of given plane in HCP unit cell (Given that ra...
Text Solution
|
- MgAl(2)O(4), is found in the Spinal structure in which O^(2-) ions con...
Text Solution
|
- Lead metal has a density of 11.34 g//cm^(3) and crystallizes in a face...
Text Solution
|
- Given that interionic distance in Na^(+), F^(-) crystal is 2.31 Å and ...
Text Solution
|
- Which of the following statement (s) for crystal having schottky defec...
Text Solution
|
- For each of the following substances, identify the intermolecular forc...
Text Solution
|
- The ZnS zinc blende structure is cubic. The unit cell may be described...
Text Solution
|
- A metal (M), shows ABAB arrangement of atoms in solid state, then what...
Text Solution
|
- A mineral of iron contains an oxide containing 72.36% iron by mass and...
Text Solution
|
- Percentage of void space in AB solid having rock salt structure if (r(...
Text Solution
|
- In an ionic solid r((+)) = 1.6 Å and r((-)) = 1.864 Å. Use the radius ...
Text Solution
|
- Ice crystallizes in a hexagonal lattice. At the low temperature at whi...
Text Solution
|
- A spinal is an important class of oxides consisting of two types of me...
Text Solution
|
- Two dimensional close packed structure can be generated by stacking th...
Text Solution
|
- Two dimensional close packed structure can be generated by stacking th...
Text Solution
|
- Two dimensional close packed structure can be generated by stacking th...
Text Solution
|