A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise Level 3|92 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise ONE OR MORE ANSWER IS/ARE CORRECT|84 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|72 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 11)|6 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-CHEMICAL BONDING (ADVANCED)-Level 2
- Which of the following molecular species is not linear?
Text Solution
|
- Incorrect match is :
Text Solution
|
- Consider the following reactions: MX(4)+X'(2) to MX(4)X(2)' If ato...
Text Solution
|
- Select the correct statements:
Text Solution
|
- In which of the following species, d-obitals having xz an yz two nodal...
Text Solution
|
- The correct order of increasing s-character (in percentage) in the hyb...
Text Solution
|
- The shapes of MnO(4)^(-) ion and the hybridization of Mn in MnO(4)^(-)...
Text Solution
|
- Which one of the following molecule will have all equal X-F bonds leng...
Text Solution
|
- Consider the following information (F=F or Cl) According to given...
Text Solution
|
- In which of the following cases C-C bond length will be highest?
Text Solution
|
- In which of the following cases C-C bond length will be highest? (I)...
Text Solution
|
- The correct order of equatorial FSF bond angle in the folllowing compo...
Text Solution
|
- Incorrect orders of bond angle is :
Text Solution
|
- Minimum F-S-F bond angle present in :
Text Solution
|
- The correct order of increasing bond angle is
Text Solution
|
- The correct order of bond anlge is :
Text Solution
|
- Which one is correct for bond angle?
Text Solution
|
- In molecules of the type AX(2)I(n) (where L represents lone pair and n...
Text Solution
|
- Which of the following solid has maximum melting points?
Text Solution
|
- The melting points of AlF(3) is 104^(@) and that of SiF(4) is -77^(@) ...
Text Solution
|
 `to` Hybridisation of `Te: sp^(3)d^(2)(sp_(x)p_(y)p_(z)d_(x^(2)-y^(2))d_(x^(2)))`
`to` Hybridisation of `Te: sp^(3)d^(2)(sp_(x)p_(y)p_(z)d_(x^(2)-y^(2))d_(x^(2)))` 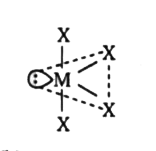 `to` Hybridisation of `Te: sp^(3)d(sp_(x)p_(y)+p_(x)d_(x^(2)))`
`to` Hybridisation of `Te: sp^(3)d(sp_(x)p_(y)+p_(x)d_(x^(2)))`