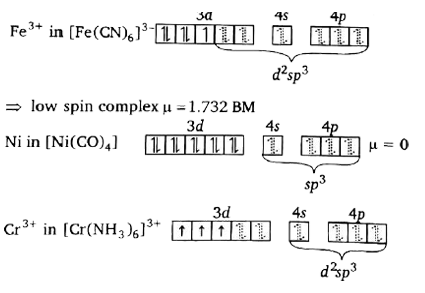
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise LEVEL 3 (PASSAGE TYPE)|97 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|26 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|43 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 11)|6 Videosd-BLOCK ELEMENTS
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|6 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-CO-ORDINATION COMPOUNDS-LEVEL 1
- Which of the following statements is correct?
Text Solution
|
- Consider that a d^(6) metal ion (M^(2+)) forms a complex with aqua lig...
Text Solution
|
- Which of the followimg complex compound(s) is/are paramagnetic and low...
Text Solution
|
- The diamagnetic species is:
Text Solution
|
- The species which has four unpaired electron is:
Text Solution
|
- Which of the following is a low spin (spin-paired) complex?
Text Solution
|
- The structure of K[PtCl(3)(C(2)H(4))] and hybridisation of Pt respecti...
Text Solution
|
- For which of the following types of ions is the number of unpaired ele...
Text Solution
|
- Among the following pairs of complexes, in which case the Delta(0) val...
Text Solution
|
- Dimethyl glyoxime forms a square planar complex with Ni^(2+) . This co...
Text Solution
|
- What is the magnetic moment ( spin only) and hybridisation of the brow...
Text Solution
|
- Choose incorrect stability order:
Text Solution
|
- Aqueous solution of Ni^(2+) contains [Ni(H(2)O)(6)]^(2+) and its magne...
Text Solution
|
- The correct order of energies of d-orbitals of metal ion in a square p...
Text Solution
|
- Which of the following is true about the complex [PtCl(2)(H(2)O)(NH(3)...
Text Solution
|
- The crystal field stabilisation energy of [Co(NH(3))(6)]Cl(3) is:
Text Solution
|
- The magnitude of crystal field stabilisation energy in octaheral field...
Text Solution
|
- Complex compound [Cr(NCS)(NH(30)(5)][ZnCl(4)] will be,
Text Solution
|
- The most stable ion is:
Text Solution
|
- In the complex K(2)Fe[Fe(CN)(6)]:
Text Solution
|