A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 114-CHEMISTRY
- When NaOH pallets are left in the open air they acquire a fluid layer ...
Text Solution
|
- Which of the following pairs of Lewis structure represent resonance co...
Text Solution
|
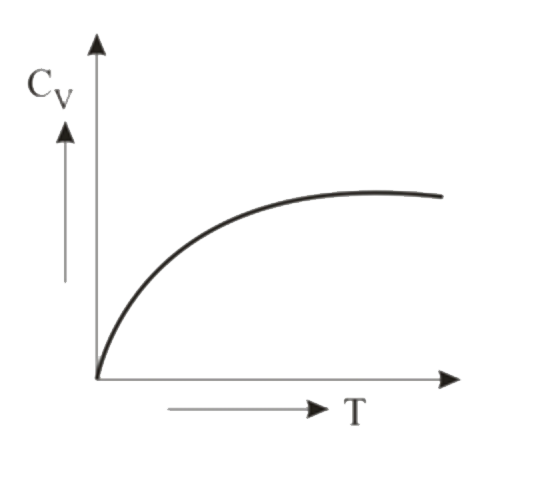
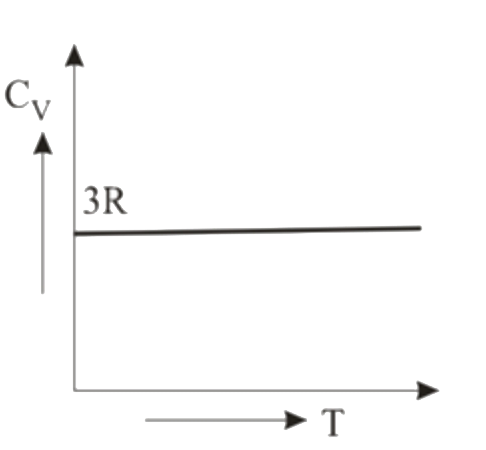
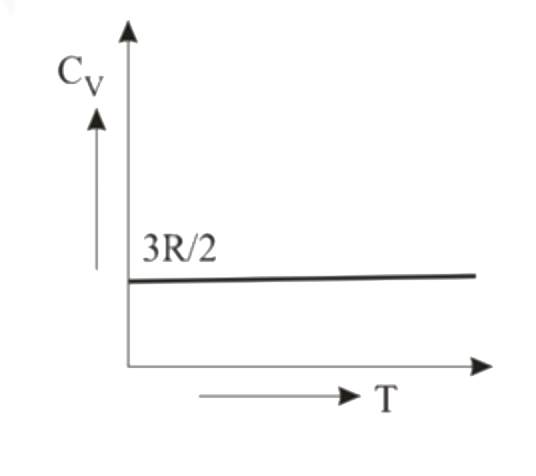
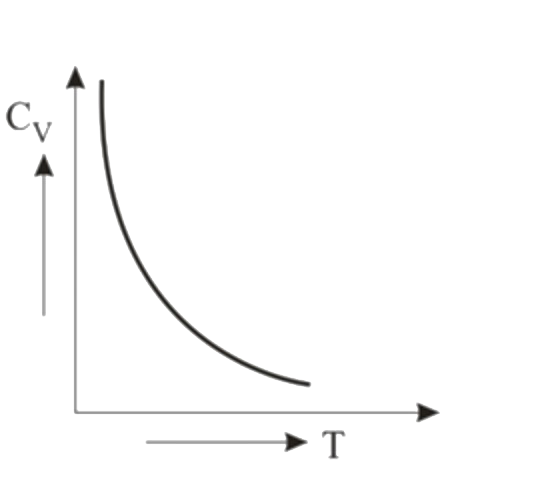
- Graph for specific heat at constant volume for a monoatomic gas
Text Solution
|
- (X), (Y), (Z) are elements in third short period. Oxide of (X) is ioni...
Text Solution
|
- On reaction of ozone with hydrogen peroxide if we start with one volum...
Text Solution
|
- The number of atoms in 100 g of an fcc crystal with density = 10.0g cm...
Text Solution
|
- Which of the following solvent will be able to dissolve dioxygen appre...
Text Solution
|
- In the given reaction (X) will be
Text Solution
|
- Which oxide of nitrogen condenses to a bluish liquid at -30^@C, but on...
Text Solution
|
- The melting point of most of the solid substances increases with an in...
Text Solution
|
- The pK(a) of acetic acid and pK(b) of ammonium hydroxide are 4.76 and ...
Text Solution
|
- The final product 'D' of the reaction
Text Solution
|
- Which of the following metal carbonyl has structure in the diagram?
Text Solution
|
- What is the final product of the reaction? (CH3)(2) = CHCH2CH(3) ove...
Text Solution
|
- An energy of 24.6 eV is required to remove one of that electrons from ...
Text Solution
|
- Given E(Ag^(o+)|Ag)^(@) = + 0.80V , E(Co^(2+)|Co)^(@) = -0.28 V, E(Cu^...
Text Solution
|
- In which of the following molecules all A - X bond lengths are identic...
Text Solution
|
- Which of the followin g orbits of hydrogen atom should have the value ...
Text Solution
|
- By starting with 0.5 moles of sodium peroxide how many moles of dioxyg...
Text Solution
|
- When mercuric iodide is added to the aqueous solution of potassium iod...
Text Solution
|