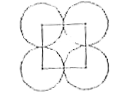A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE SOLID STATE
AAKASH INSTITUTE|Exercise Assignment (SECTION - C) (PREVIOUS YEARS QUESTION)|41 VideosTHE SOLID STATE
AAKASH INSTITUTE|Exercise Assignment (SECTION - D) (ASSERTION-REASON TYPE QUESTION)|20 VideosTHE SOLID STATE
AAKASH INSTITUTE|Exercise Assignment (SECTION - A) (OBJECTIVE TYPE QUESTION)|58 VideosTHE S-BLOCK ELEMENTS
AAKASH INSTITUTE|Exercise Assignment (Section-J)|10 VideosTHERMODYNAMICS
AAKASH INSTITUTE|Exercise ASSIGNMENT (Section -D) Assertion-Reason Type Questions|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-THE SOLID STATE -Assignment (SECTION - B) (OBJECTIVE TYPE QUESTION)
- In a unit cell, atoms A, B, C and D are present at corners, face - cen...
Text Solution
|
- In a CsCl structure, if edge length is a, then distance between one Cs...
Text Solution
|
- The correct statement about, CCP structure is
Text Solution
|
- In a NaCl structure, if positions of Na atoms and Cl - atoms are inter...
Text Solution
|
- If radius of a metal atom (A) is 5pm and radius of an electronegative ...
Text Solution
|
- A metal can be crystallized in both BCC and FCC unit cells whose edge ...
Text Solution
|
- In a unit cell containing X^(2+), Y^(3+) and Z^(2-) where X^(2+) occu...
Text Solution
|
- On rising temperature and decreasing pressure in CsCl solid
Text Solution
|
- In a ccp type structure, if half of atoms are removed, then percentag...
Text Solution
|
- In a BCC unit cell, if half of the atoms per unit cell are removed, th...
Text Solution
|
- Number of atoms per unit cell, if atoms are present at the corner of u...
Text Solution
|
- Number of unit cells in 10 g NaCl
Text Solution
|
- Some of the molecular solid upon heating produces small amount of elec...
Text Solution
|
- NaCl becomes paramagnetic at high temperature due to
Text Solution
|
- How many Cs^+ ions occupy the second nearest neighbour location of a C...
Text Solution
|
- The ratio of number of rectangular plane and diagonal plane in a cubic...
Text Solution
|
- In the calcum fluaride structure, the coordination bumber of the catio...
Text Solution
|
- which of the following defects decrase the density decrease the d...
Text Solution
|
- Glass is a
Text Solution
|
- The packing efficiency of the 2D square unit cell shown below is
Text Solution
|