Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Multiple choice question|26 VideosSTRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Praticle based question|23 VideosSTRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Long Answer Question|20 VideosMATTER IN OUR SURROUNDINGS
DINESH PUBLICATION|Exercise (LAQs) Long Answer Questions|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-STRUCTURE OF ATOM -Higher order thinking skilll based questions
- Both helium (He) and beryllium (Be) have two valence electrons.Whereas...
Text Solution
|
- Study the data given below answer the qusestion which follow: (i)...
Text Solution
|
- Which of the two will be chemically more reactive , element x with at...
Text Solution
|
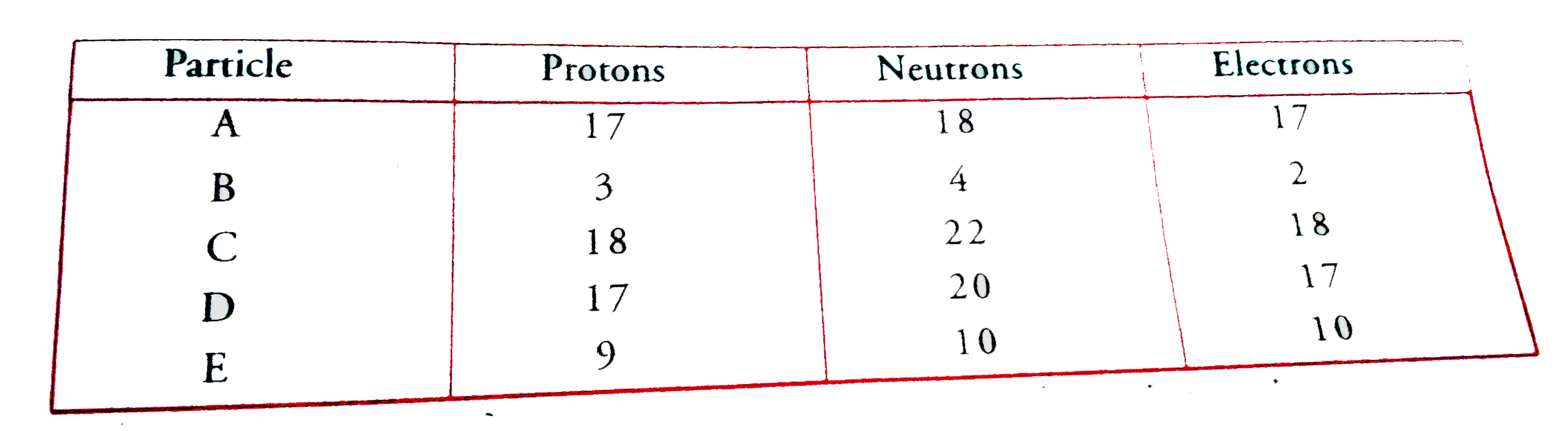
- The number of protons neuitrons and electrons in particles from A to E...
Text Solution
|
- An atom of an elemets has three elctons in the third shell which is th...
Text Solution
|