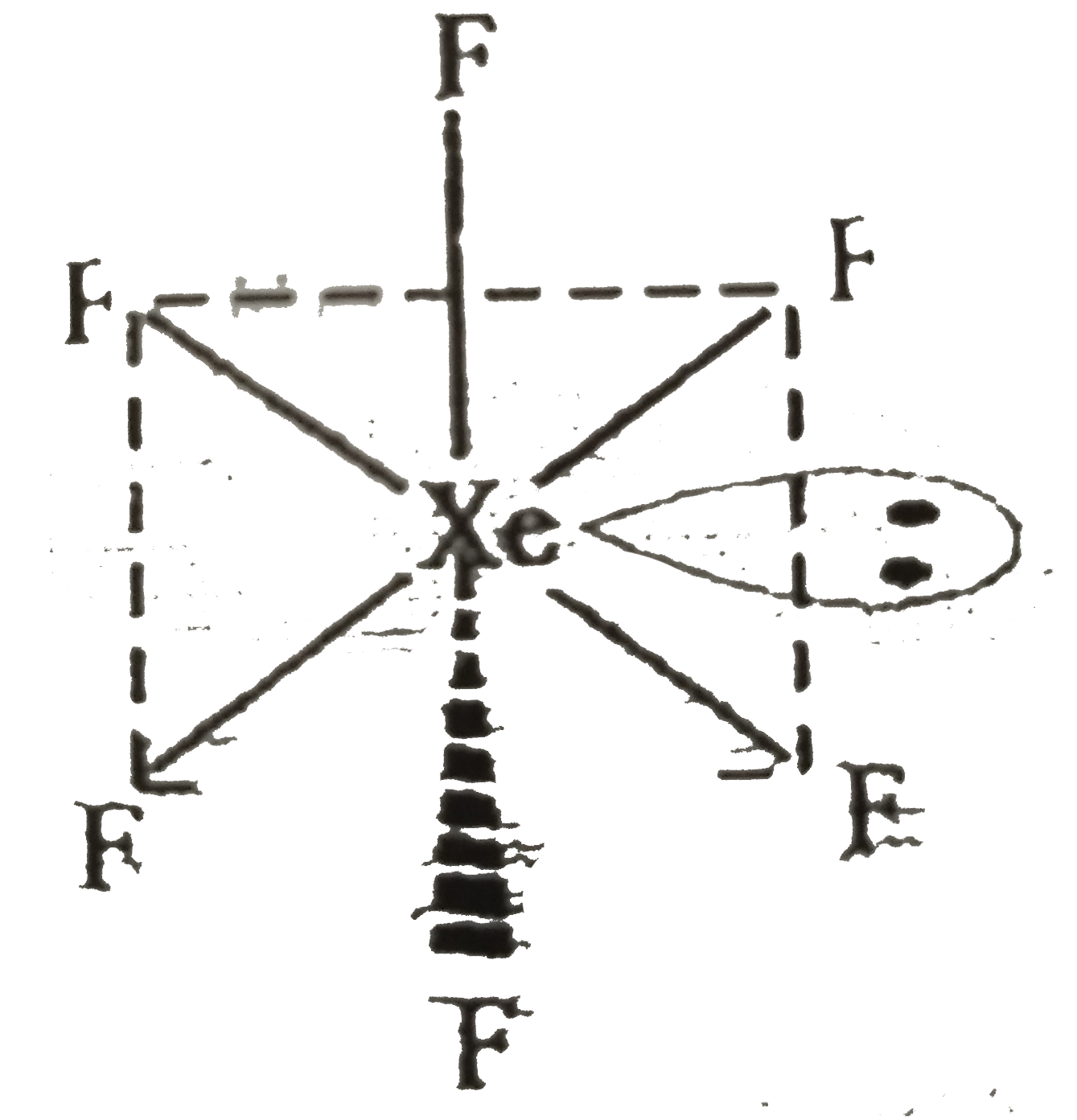
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- Hybridisation is a theoretical concept, as state of hybridisation can ...
Text Solution
|
- Hybridisation is a theoretical concept, as state of hybridisation can ...
Text Solution
|
- Hybridisation is a theoretical concept, as state of hybridisation can ...
Text Solution
|
Text Solution
|
- When heated, lithium reacts with nitrogen to gorm lithium nitride: L...
Text Solution
|
- From the graph of (d)/(P) vs P at a constant temperature of 300K. Calc...
Text Solution
|
- (W) 4H(3)PO(4)overset(-3H(2)O)rarr Compound 'X' How may P-O-P linkage ...
Text Solution
|
- To find formulae of a compound if we titrate NH(3) in the compound wit...
Text Solution
|
- How many different type of functional groups are presetn in following ...
Text Solution
|
- At 727^(@)C and 1.2 atm of total equilibrium pressure, SO(3) is partia...
Text Solution
|
- Which of the following has sp^(3)d^(3) hybridisation?
Text Solution
|
- At a certain temperature T, a compound AB(4)(g) dissociates as 2AB(4)(...
Text Solution
|
- The air contain 80% N(2) and 20% O(2) by volume. The volume occupied b...
Text Solution
|
- When dilute H(2)SO(4) is electrolysed by using platinum electrodes the...
Text Solution
|
- The angular momentum of an electron in a certain orbit of Li^(+2) ion ...
Text Solution
|
- As the temperature is raised from 20^(@)C to 40^(@)C the averge kineti...
Text Solution
|
- A^(+n)(g)overset(x)rarrA^(+(n+1))(g)+e^(-) In above process 'X' is
Text Solution
|
- The distance of closest approach of an alpha-particle fired towards a ...
Text Solution
|
- The sealed containers of same capacity and at the same temperature and...
Text Solution
|
- A mixture of equal mass of O(2) and O(3) gases are allowed to effuse t...
Text Solution
|