A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- A gas has been subjected to a cycle of isochoric, isothermal process 1...
Text Solution
|
- If our eye receive a signal consisting of light having wavelengths lam...
Text Solution
|
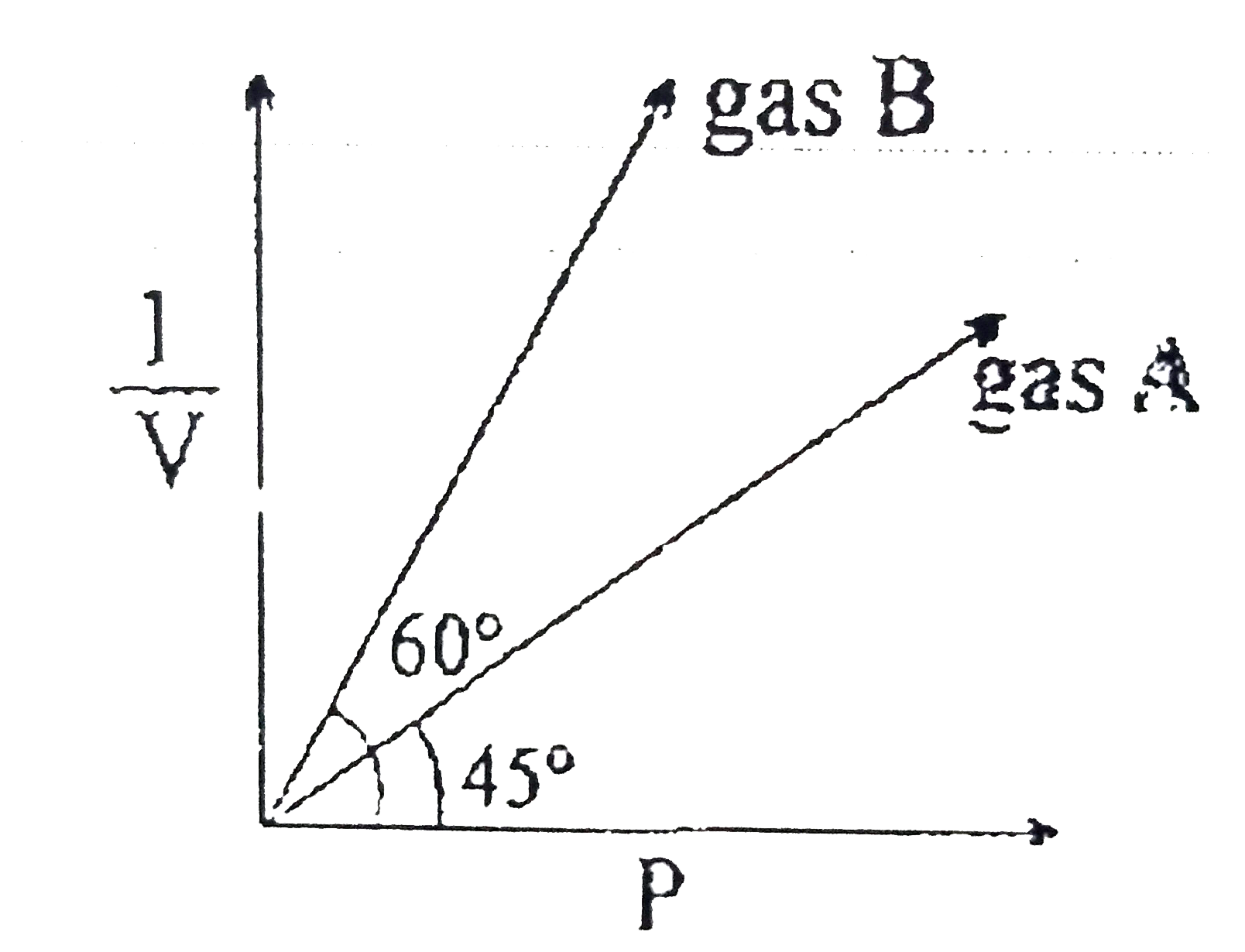
- At constant temperature of 273K. (1)/(v) vs are plotted for two ideal ...
Text Solution
|
- How many maximum number of atoms are present in signle plane of Al(CH(...
Text Solution
|
- Statement-1: Hybridisation is the mixing of atomic orbitals. Stateme...
Text Solution
|
- Statement-1: In all hydrogen halide compound HF is the only liquid aci...
Text Solution
|
- Which of the following regents are used to remove hardness present in ...
Text Solution
|
- Select the correct statements regarding Na(6)P(6)O(18) compound:
Text Solution
|
- If 4 litre of H(2) gas at 400 mm Hg and 47^(@)C is transferred to 19 l...
Text Solution
|
- Which resonating structure is most stable?
Text Solution
|
- How many total number of electrons have m=01 value in only those orbit...
Text Solution
|
- If 10gm of water4 is added to 150gm of oleum (104.5%), then the find s...
Text Solution
|
- Which of the following is aromatic?
Text Solution
|
- K(2)Cr(2)O^(7)+H(2)SO(4)+4H(2)O(2)rarr underset("Suphur compound")X+...
Text Solution
|
- Consider the following equilibrium H(2)O(g)+CO(g)hArrH(2)(g)+CO(2)(g...
Text Solution
|
- Approximate De-Brogile wavelength ratio of alpha particle with respect...
Text Solution
|
- Which is not valid resonating structure of H(2)C=CH-CH=CH-O-CH(3)
Text Solution
|
- Species which is most reactive in among the following-
Text Solution
|
- For the reaction at equilibrium: A(g)+2B(g)hArrC(g) Equilibrium c...
Text Solution
|
- Which order is correct for bond length for compound?
Text Solution
|