A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- Which of the following is/are aromatic?
Text Solution
|
- Which of the following compound(s) has/have two delocaisedion pair?
Text Solution
|
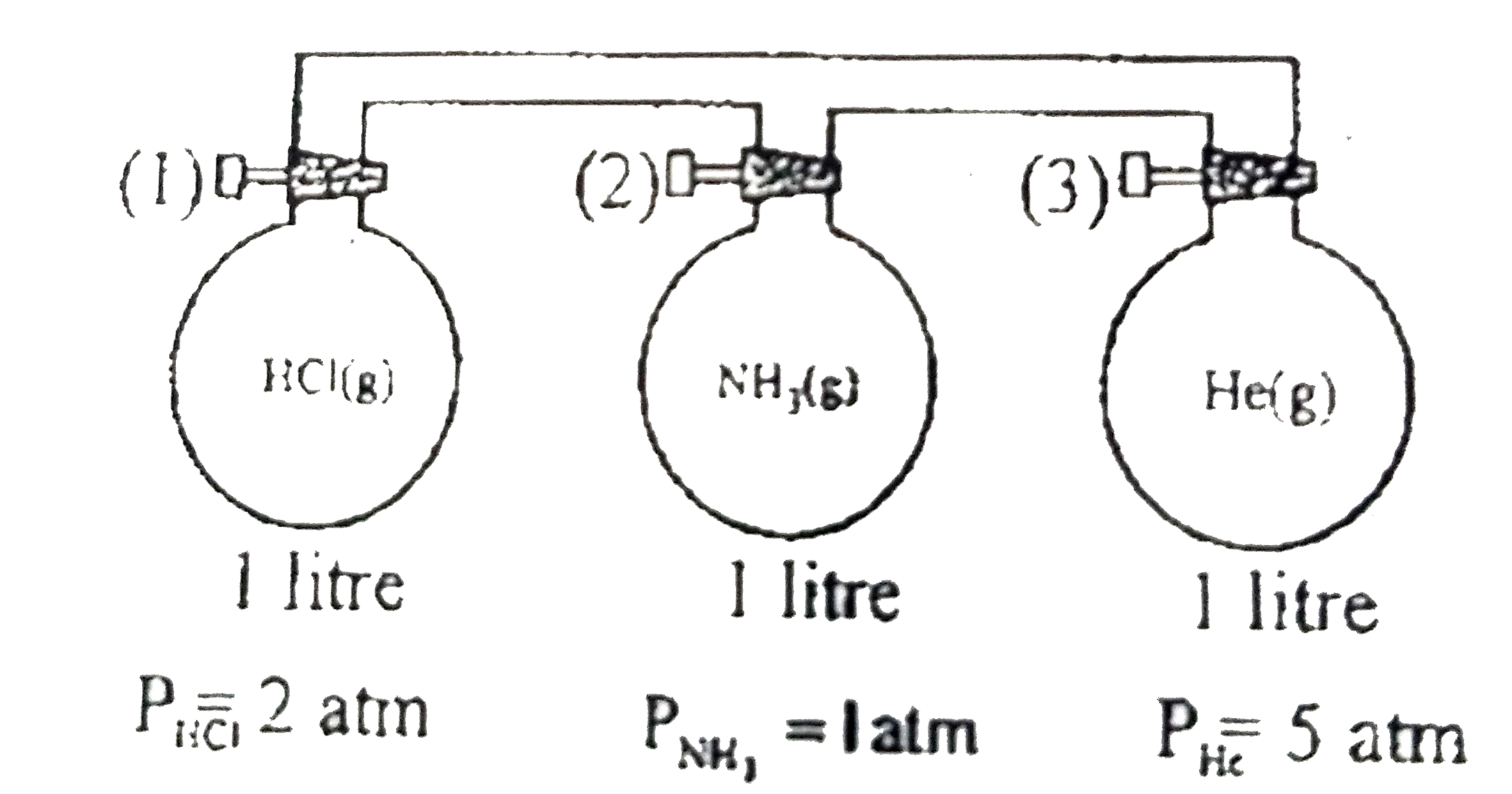
- Consider three flasks in diagram below. Assuming that connecting tube ...
Text Solution
|
- Consider three flasks in diagram below. Assuming that connecting tube ...
Text Solution
|
- If the quantum numbers n,l,m and s were defined as: R=shell number ...
Text Solution
|
- If the quantum numbers n,l,m and s were defined as: R=shell number ...
Text Solution
|
- If the concentration of Mg^(2+) ions in sea water is 1200 ppm. How man...
Text Solution
|
- Amongest the following, the total number of compounds whose aqueous so...
Text Solution
|
- 4.6 gm of liquid ethanol (C(2)H(5)OH) is taken in 12 litre container a...
Text Solution
|
- Consider following carbanions give write number of catbonions which ar...
Text Solution
|
- Gold from Gold bearing rock can be dissolved with NaCN in presence of ...
Text Solution
|
- Using option how many total number of statements are correct. (I) H-...
Text Solution
|
- Diamond structure can be considered as ZnS (Zinc blend) structure in w...
Text Solution
|
Text Solution
|
Text Solution
|
- Selecthe correct statement(s):
Text Solution
|
- Give the conect order of initials T(true) or F(false) for following st...
Text Solution
|
- In correct statement about given carbohydrate is
Text Solution
|
- Correct statement about I and II
Text Solution
|
- HgI2 (yellow) will be turned into Hgl2 (med) variety on
Text Solution
|