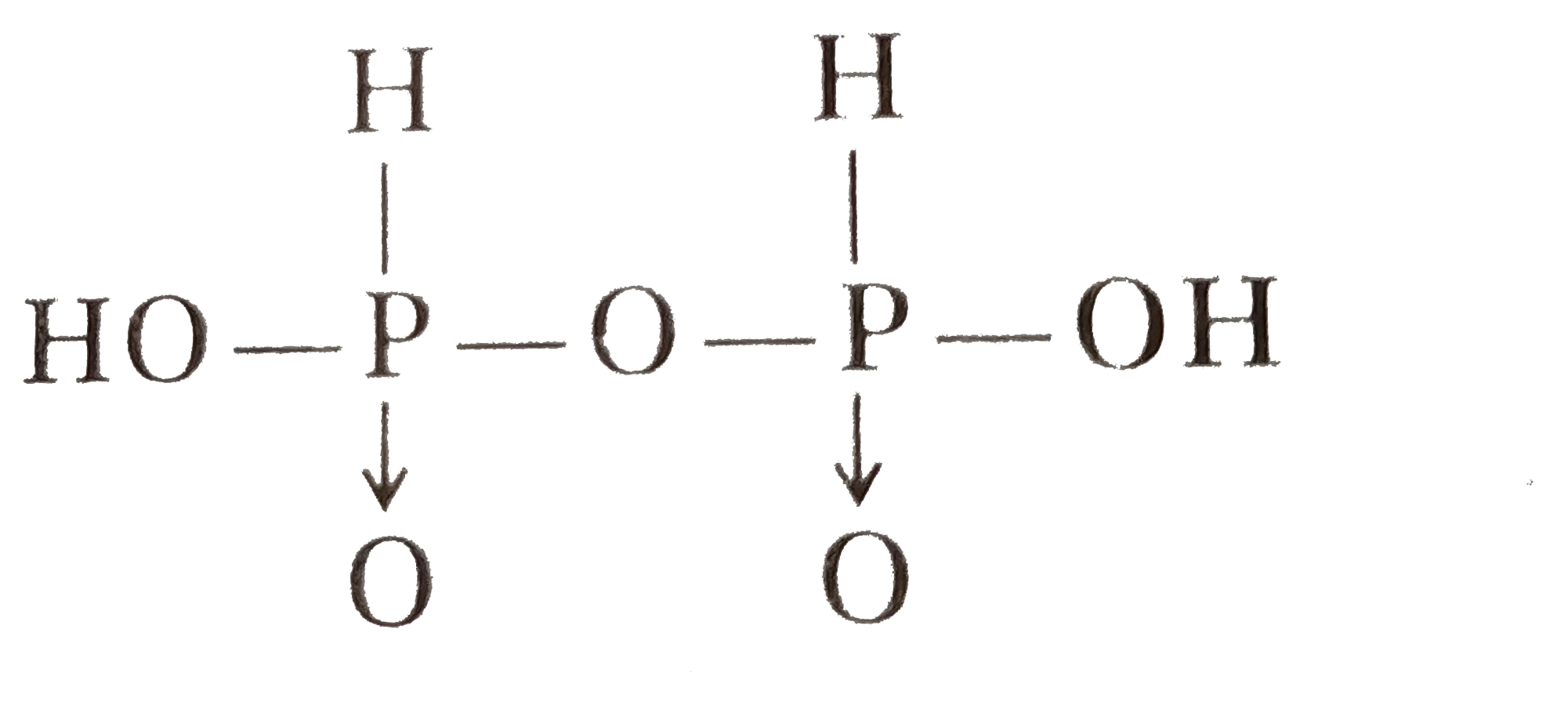A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
CHHAYA PUBLICATION|Exercise MCQ HOTSPOT (SINGLE CORRECT TYPE)|40 VideosREDOX REACTIONS
CHHAYA PUBLICATION|Exercise MCQ HOTSPOT (MORE THAN ONE CORRECT TYPE)|21 VideosREDOX REACTIONS
CHHAYA PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS (HOTS) QUESTIONS|12 VideosCLASSIFICATION OF ELEMENTS & PERIODICITY IN PROPERTIES
CHHAYA PUBLICATION|Exercise PRACTICE SET|12 Videoss-BLOCK ELEMENTS
CHHAYA PUBLICATION|Exercise PRACTICE SET|16 Videos
Similar Questions
Explore conceptually related problems
CHHAYA PUBLICATION-REDOX REACTIONS -ENTRANCE QUESTION
- The equivalent weight of K(2)Cr(2)O(7) in acidic medium is expressed i...
Text Solution
|
- If Cl(2) is passed through hot aqueous hot NaOH, the products formed h...
Text Solution
|
- In aqueous alkaline solution, two electron reduction of HO2^- gives
Text Solution
|
- Consider the following reaction XMNO(4)^(-)+yC2O(4)^(2-)+zH^(+)toxMn^(...
Text Solution
|
- In which of the following reactions, H(2)O(2) acts as a reducing agent...
Text Solution
|
- The pair in which phosphorus atoms have a formal oxidation state of +...
Text Solution
|
- Which of the following reactions is an example of a redox reaction -
Text Solution
|
- The oxidation states of Cr in [Cr(H(2)O)(6)]CI(3)[Cr(C(6)H(6))(2)] and...
Text Solution
|
- A mixture of potassium chlorate ,oxalic acid and sulphuric acid is hea...
Text Solution
|
- In which of the following compounds ,nitrogen exhibits highest oxidati...
Text Solution
|
- In acidic medium H(2)O(2) changes Cr(2)(7)^(2-)toCrO(5)which has two (...
Text Solution
|
- (I) H(2)O(2)+O(3)toH(2)O+2O(2) (II) H(2)O(2)+Ag(2)Oto2Ag+H(2)O+O(2) ...
Text Solution
|
- Assuming complete inoisation ,same moles of which of the following com...
Text Solution
|
- Hot concentrated sulphuric acid a is a moderately strong oxidising age...
Text Solution
|
- For the redox reaction - MnO(4)^(-)+C(2)O(4)^(2-)+H^(+)toMn^(2+)+CO(2...
Text Solution
|
- When KMnO(4) reacts with KBr in alkaline medium and gives bromate ion,...
Text Solution
|
- K(2)Cr(2)O(7) in acidic medium coverts into-
Text Solution
|
- Oxidation state of iron in haemoglobin is-
Text Solution
|
- What is the oxidation number of Br in KBrO(4)^
Text Solution
|
- Substances that are oxidised and reduced in the following reaction are...
Text Solution
|