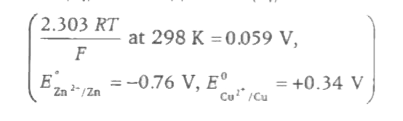A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-AP EAMCET ENGINEERING ENTRANCE EXAM ONLINE QUESTION PAPER 2019 (SOLVED)-PHYSICS
- The number of moles of solute present in the solutions of I, II and II...
Text Solution
|
- 6g of a mixture of napthalene (C10H8) and anthracene (C14H10) is disso...
Text Solution
|
- Under which of the following conditions E value of the cell, for the c...
Text Solution
|
- In the first order thermal decomposition of C2H5I(g) to C2H4 (g) + HI(...
Text Solution
|
- Identify the correst statements from the following: I. Sulphur sol i...
Text Solution
|
- Which of the following statements is not correct? I. Magneitite II. ...
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- Noble metals, like gold and platinum are soluble in which of the follo...
Text Solution
|
- Identify the set of acidic oxides.
Text Solution
|
- The wavelengths of light absorbed by the complexes [Ni(H2O)6]^(2+),[...
Text Solution
|
- KMnO4 oxidises S2O3^(2-) to SO4^(2-) in medium x and NO2^(-) to NO3^(...
Text Solution
|
- Match the following:
Text Solution
|
- Identify the correct set of monosaccharides present in sucrose (X), la...
Text Solution
|
- Which of the following are broad spectrum antibiotics?
Text Solution
|
- Arrange of following organic halides in correct order of reactivity to...
Text Solution
|
- The bond angle between C-O and O-H bonds in alcohols is close to
Text Solution
|
- Identify Z in the following sequence of reactions.
Text Solution
|
- Identify X,Y,Z in the following reactions sequence.
Text Solution
|
- 2-methyl-2 butane on hydration gave an alcohol X. Isomers of X could b...
Text Solution
|
- Acetic acid on heating with NH3 forms A. When A reacts with LiAH4 foll...
Text Solution
|