A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-QUESTION PAPER 2015-CHEMISTRY
- On the top of a mountain water boils at
Text Solution
|
- Which one of the following is the wrong statement about the liquid?
Text Solution
|
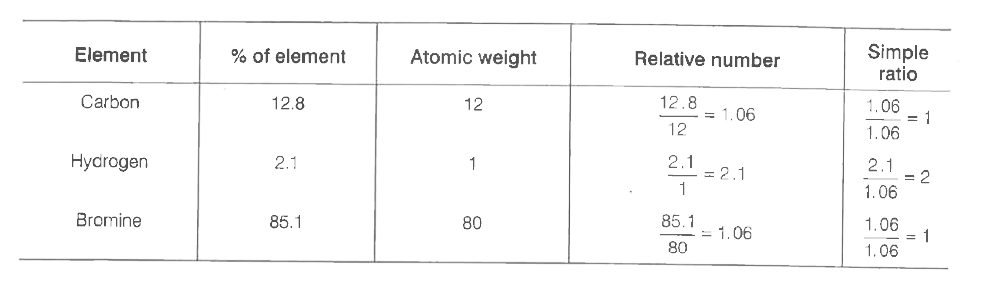
- A carbon compound contains 12.8% Carbon, 2.1% Hydrogen, 85.1% Bromine....
Text Solution
|
- 3.011 xx 10^(22) atoms of an element weighs 1.15 g. The atomic mass of...
Text Solution
|
- Which of one following is applicable is applicable for an adiabatic ex...
Text Solution
|
- On increasing temperature, the equilibrium constant of exothermic and ...
Text Solution
|
- What is the pH of the NaOH solution when 0.04 g of it is dissolved in ...
Text Solution
|
- Which of the following methods is used for the removal of temporary ha...
Text Solution
|
- Assertion (A): Alkali metals are soft and have low Melting and boiling...
Text Solution
|
- identify the correct statement
Text Solution
|
- Assertion (A): Noble gases have very low boiling points. Reason (R):...
Text Solution
|
- Which of the following statements are correct? A. Ocean is sink for CO...
Text Solution
|
- The bond angle in methoxy methane is
Text Solution
|
- Which of the following compounds has zero dipole moment?
Text Solution
|
- Which of the following reagent is used to find out carbon-carbon multi...
Text Solution
|
- Pure silicon doped with phosphorus is
Text Solution
|
- 18 g of glucose is dissolved in 90 g of water. The relative lowering o...
Text Solution
|
- A gas 'X' is dissolved in water at '2' bar pressure. Its mole fraction...
Text Solution
|
- Calculate Delta G^(@) for the following cell reaction. Zn((s)) + Ag(2)...
Text Solution
|
- The time required for a first order reaction to complete 90% is T. Wha...
Text Solution
|