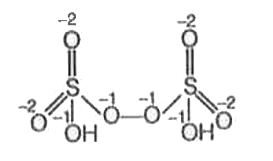
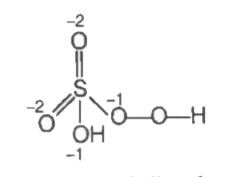
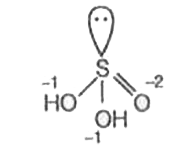
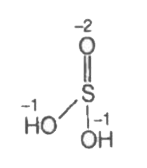
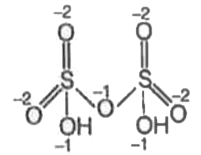
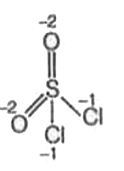
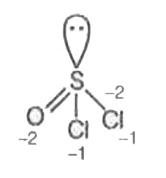
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-AP EAMCET ENGINEERING ENTRANCE EXAM ONLINE QUESTION PAPER 2019 (SOLVED)-Chemistry
- Observe the following molecules : PCl(5) , BrF(5), ClF(5), PF(5)C...
Text Solution
|
- If the kinetic energy and RMS speed of a gas at a certain temperature...
Text Solution
|
- In how many of the following compounds of sulphur, the oxidation state...
Text Solution
|
- What is the nature of reaction at 298 K, if the entropy change and e...
Text Solution
|
- The value of K(C) for the equilibrium reaction CO(2)(g) +C(s)hArr 2...
Text Solution
|
- 50 mL of 0 . 02 M Na OH solution is mixed 50 mL of 0 . 6 M acetic acid...
Text Solution
|
- Which of the following is water -gas shift reaction ?
Text Solution
|
- Magnesium is burnt in air to form A nd B . When B is hydrolysed , C an...
Text Solution
|
- Which of the following reactions can be used to prepare diborane ? ...
Text Solution
|
- Identify the correct staements . I. Germanium exists only in trace...
Text Solution
|
- H(2)C = CH(2) +O(2) overset("Aqueous medium")to CH(3) CHO What is ...
Text Solution
|
- In the following resonance structures the curved arrow indicates that ...
Text Solution
|
- In the detection of nitrogen of an organic compound by Lassaigne's tes...
Text Solution
|
- Identify Z in the following sequence of reactions C(2)H(2)(2) Br u...
Text Solution
|
- Which one of the following is not present in the nitration mixture ?
Text Solution
|
- A compound is formed from elements X and Y.. The atoms of Y (anions) f...
Text Solution
|
- The vaporu pressures of chloroform (CHCl(3)) , dichloromethane (CH(2)...
Text Solution
|
- The elevation in boling point of an aqueous solutions of NaCl is 0.01 ...
Text Solution
|
- CuSO(4) solution is electrolysed for 15 minutes to deposity 0 . 4725...
Text Solution
|
- The rate constants for a reaction at 400 K and 500 K are 2 . 60 xx 1...
Text Solution
|