A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-AP EAMCET ( ONLINE QUESTION PAPER 2018 SOLVED)-CHEMISTRY
- The bond dissociation energy ( E) and bond length ( R) of O(2),N(2) an...
Text Solution
|
- If the RMS speed of nitrogen at a certain temperature is 3000 ms^(-1) ...
Text Solution
|
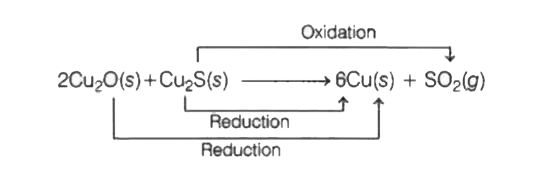
- 2Cu(2)O(s)+Cu(2)(s) rarr 6Cu(s) +SO(2)(g) the oxidant and reductant re...
Text Solution
|
- If 1.5 L of an ideal gas at a pressure of 20 atm expands isothermally ...
Text Solution
|
- At T (K) the equilibrium constant of H(2)(g)+I(2)(g) rarr 2HI(g) is 4...
Text Solution
|
- If the pH of 0.10 M monobasic acid at 298 K is 5.0 the value the value...
Text Solution
|
- Identify the correct statements from the following . (i) The number ...
Text Solution
|
- Observe the following compounds. (i) CaCO(3) " " (ii) MgSO(4)...
Text Solution
|
- A few grams of borax is dissolved in distilled water . The pH range of...
Text Solution
|
- An element (X) when burnt in oxygen forms neutral XO and acidic XO(2)....
Text Solution
|
- Match the following The correct answer is
Text Solution
|
- In the following three dimensional structure of CH(4) the bods are lab...
Text Solution
|
- Identify X and Y in the following reactions
Text Solution
|
- Which one of the following has highest dipole moment ?
Text Solution
|
- If the side length of a face centered unit cell of a metal is 400 pm a...
Text Solution
|
- If CO(2) gas having a partial pressure of 1.67 bar is bubbled through ...
Text Solution
|
- 12.25 g of CH(3)CH(2)CHCICOOH is added to 250 g of water to make a sol...
Text Solution
|
- Assertion (A) : The charge on one mole of electrons is one Faraday. ...
Text Solution
|
- If the half lives of the first order reaction at 350 K and 300 K are 2...
Text Solution
|
- Which one of the following is present in gas mask?
Text Solution
|