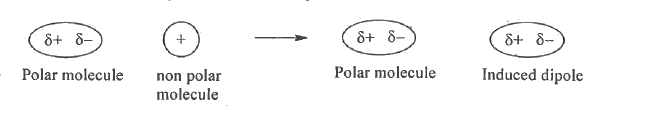Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
SUBHASH PUBLICATION|Exercise THREE MARK QUESTIONS AND ANSWERS|9 VideosSTATES OF MATTER : GASES AND LIQUIDS
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS|25 VideosSTATES OF MATTER : GASES AND LIQUIDS
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS|25 VideosSOME BASIC CONCEPTS OF CHEMISTRY
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS:|49 VideosSTRUCTURE OF ATOM
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS|29 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS -TWO MARK QUESTIONS AND ANSWERS
- Why ethyl alcohol 10 Ower boiling point than water ?
Text Solution
|
- A human adult breathes in apporximately 0.50 L of atm with each breath...
Text Solution
|
- Briefly explain dipole-induced-dipole interaction with example.
Text Solution
|
- Briefly explain London or disperison forces
Text Solution
|
- Explain graphical representation of Boyle's law on effect of pressure ...
Text Solution
|
- Explain graphical representation of Charle's law on effect of volume v...
Text Solution
|
- Calculate the total pressure in a 10 L cylinder which contains 0.4 g ...
Text Solution
|
- Calculate the number of moles of hydrogen gas at a pressure of 760 mm ...
Text Solution
|
- 34.95 ml of phosphorus vapour weighs 0.0625g at 0.1 bar pressure. Wha...
Text Solution
|
- In terms of Charle's law explain why b-273^(@)C is the lowest possible...
Text Solution
|
- Why is Boyle's law is obyed by N(2), O(2) or CO(2) ony at low pressur...
Text Solution
|
- Compare the rate of diffusion of HCl and NH(3) (Atomic massses of H1u,...
Text Solution
|
- State Avogadro law and write mathematical form.
Text Solution
|
- What is STP ?
Text Solution
|
- Derive ideal gas equation from gas laws.
Text Solution
|
- Calculate gas constant (R) value in litre. Bar. /K/ mole.
Text Solution
|
- Calculate gas constant (R) value in litre. Bar. /K/ mole.
Text Solution
|
- Derive the relation between Density and Molar mass of a gaseous substa...
Text Solution
|
- State Dalton's law of partial pressure and write mathematical form.
Text Solution
|
- Explain Aqueous tension.
Text Solution
|