Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS|25 VideosSTATES OF MATTER : GASES AND LIQUIDS
SUBHASH PUBLICATION|Exercise TWO MARK QUESTIONS AND ANSWERS|34 VideosSOME BASIC CONCEPTS OF CHEMISTRY
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS:|49 VideosSTRUCTURE OF ATOM
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS|29 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS -THREE MARK QUESTIONS AND ANSWERS
- Write the postulates of kinetic theory of gases.
Text Solution
|
- Discuss the intermolecular forces v/s thermal interaction.
Text Solution
|
- What are the characters of gasesous state.
Text Solution
|
- Explain Gay Lussac's law.
Text Solution
|
- Derive the relationship between partial pressure of gas and its mole f...
Text Solution
|
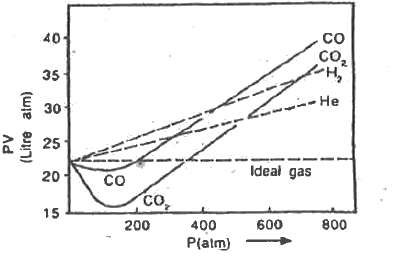
- Mention of causes for the deviation of ral gas from ideal behaviour.
Text Solution
|
- Explain the significance of compressibility factor.
Text Solution
|
- (i) What is he effect of temperature on (a) density, (b) surface tensi...
Text Solution
|
- The pressure of real gases is less than that of ideal gas because of
Text Solution
|