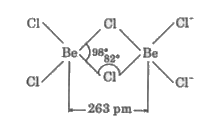
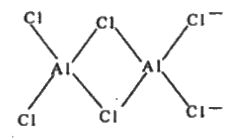
Text Solution
Verified by Experts
Topper's Solved these Questions
S-Block Elements
SUBHASH PUBLICATION|Exercise Three marks questions and answers|8 VideosS-Block Elements
SUBHASH PUBLICATION|Exercise Three marks questions and answers|8 VideosREDOX REACTIONS
SUBHASH PUBLICATION|Exercise Three Marks Questions|29 VideosSOME BASIC CONCEPTS OF CHEMISTRY
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS:|49 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-S-Block Elements-Two marks questions and answers
- What are the properties of calcium oxide?
Text Solution
|
- Which metals is present in chlorophyll? How does this metals react wit...
Text Solution
|
- Name an alkali metal carbonate which is thermally ustable and why? Giv...
Text Solution
|
- Why are ionic hydrides of only alkali metals and alkaline earth metals...
Text Solution
|
- Which one of the alkaline earth metals carbonate is thermally most and...
Text Solution
|
- Which out of LI, Na, K, Be, Mg, Ca has lowest ionization enthalpy and ...
Text Solution
|
- Which alkali metal ion forms largest hydrate ion in aqueous solution a...
Text Solution
|
- What is responsible for the blue colour of the solution of alkali meta...
Text Solution
|
- Heat of Hydration of Na^(+) (size 102 Pm) = - 397J Kj mol^(-1) whereas...
Text Solution
|
- Discuss the diagonal relationship of Be and AI with regard to (i) acti...
Text Solution
|
- How to prepare calcium carbonate (Lime stone) from slaked lime ?
Text Solution
|
- Explain the biological importance of magnesium and calcium.
Text Solution
|
- Lithium is kept wraped in parafin wax not stored in kerosene oil. Why?
Text Solution
|
- When lithium is heated in air mainly forms the monoxide and not peroxi...
Text Solution
|
- What is the colour imparts when calcium, Barium and strontium undergoe...
Text Solution
|
- What is the formula of gypsum? What happens when it is heated?
Text Solution
|
- Beryllium and magnesium do not impart colour to bunsen flame. Why?
Text Solution
|
- Which colour is imparted when Lithium, sodium, potassium and Rubidiumk...
Text Solution
|
- The ionic compounds of alkali metals colourless. Why?
Text Solution
|
- Alkali metals are good conductors of electricity. Why?
Text Solution
|