Text Solution
Verified by Experts
Topper's Solved these Questions
SUPER MODEL QUESTION PAPER (FOR ANSWER)
SUBHASH PUBLICATION|Exercise PART-D|28 VideosSUPER MODEL QUESTION PAPER (FOR ANSWER)
SUBHASH PUBLICATION|Exercise PART-E|19 VideosSUPER MODEL QUESTION PAPER (FOR ANSWER)
SUBHASH PUBLICATION|Exercise PART-B|14 VideosSTRUCTURE OF ATOM
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS|29 VideosSUPER MODEL QUESTION PAPER (FOR PRACTICE)
SUBHASH PUBLICATION|Exercise PART-E|17 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-SUPER MODEL QUESTION PAPER (FOR ANSWER)-PART-C
- Mention one significance for each of the four quantum numbers.
Text Solution
|
- Write the energy level diagram of oxygen molecule.
Text Solution
|
- Calculate the heat of reaction of the following reaction C(6)H(12)O(6(...
Text Solution
|
- A buffer solution of pH 8.3 is prepared from ammonium chloride and amm...
Text Solution
|
- A colourless liquid A contains H and O elements only. It decomposes sl...
Text Solution
|
- Give three uses each of the different allotropic forms of carbon.
Text Solution
|
- Explain the mechanism of halogenation or chlorination of benzene.
Text Solution
|
- Name the product obtained when Benzene is hydrogenated in presence of ...
Text Solution
|
- 4 g of copper chloride on analysis was founded to contain 1.890 g of c...
Text Solution
|
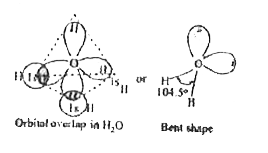
- Explain the structure of water molecule ?
Text Solution
|
- Explain th partical nature of EMR.
Text Solution
|
- Define reduction in terms of electronic concepts?
Text Solution
|
- Define cathode and anode.
Text Solution
|
- Give the IUPAC name of the following compounds.
Text Solution
|
- In Leibig's method 0.24 g of organic compound on combustion with dry o...
Text Solution
|
- Why does benzene undergo electrophilic substituion easily and nucleoph...
Text Solution
|
- Write the balanced chemical equations for the combustion of the follow...
Text Solution
|