Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-PUE DEPT. MODEL QUESTION PAPER-PART-C
- A compound contains 4.07% hydrogen, 24.27% Carbon and 71.65%, Chlorine...
Text Solution
|
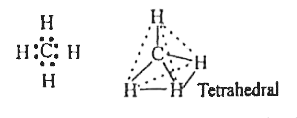
- Expain the formation of methane molecule on the basis of hybridization...
Text Solution
|
- Between O(2) and O(2)^(-) which one has higher bond order?
Text Solution
|
- What is Oxidation Number? What is the oxidation Number of Cl is KClO(3...
Text Solution
|
- Write separate equations for the oxidation and reduction reactioins oc...
Text Solution
|
- What are Isothermal and Adiabatic process?
Text Solution
|
- State the II law of Thermodynamics. Give the equation that relates Gib...
Text Solution
|
- Giving justification, catogories the following species as Nuclophile o...
Text Solution
|
- Mention the differences between Fractional distillation and Steam Dist...
Text Solution
|
- Write the IUPAC names of the following compounds.
Text Solution
|
- Explain position isomerism with example.
Text Solution
|
- Given equations for the reactions that occurs when Ehyene is treated...
Text Solution
|
- Given equations for the reactions that occurs when Eenthyene is trea...
Text Solution
|
- Explain Friedel -Craft's acylation with an example.
Text Solution
|