Text Solution
Verified by Experts
Topper's Solved these Questions
PUE DEPT. MODEL QUESTION PAPER
SUBHASH PUBLICATION|Exercise PART-E|13 VideosPUE DEPT. MODEL QUESTION PAPER
SUBHASH PUBLICATION|Exercise PART-A|11 VideosPUE DEPT. MODEL QUESTION PAPER
SUBHASH PUBLICATION|Exercise PART -C|16 VideosP-BLOCK ELEMENTS
SUBHASH PUBLICATION|Exercise Three mark questions and answers|24 VideosREDOX REACTIONS
SUBHASH PUBLICATION|Exercise Three Marks Questions|29 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-PUE DEPT. MODEL QUESTION PAPER-PART-D
- Acetic acid has a dissociation constant of 1.8xx10^(-5), Calculate the...
Text Solution
|
- Calculate the ionization constant of 0.05M weak acid if it's degree of...
Text Solution
|
- Describe LCAO method for the formation molecular orbitals of Hydrogen....
Text Solution
|
- Give two difference between bonding molecular orbital and antibonding ...
Text Solution
|
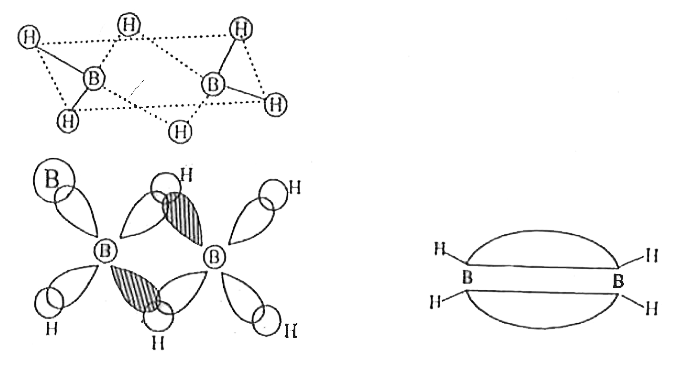
- What is the formulae of inorganic benzene? Write the structure of Dibo...
Text Solution
|
- What are Fullurenes? How are they propared?
Text Solution
|
- Write the principles involved in the estimation of (i) Halogens (ii) S...
Text Solution
|
- Name the element estimated by Kjeldhal's method.
Text Solution
|
- Calculate the mole fraction of Ethanol (C(2)H(5)OH) in the solution co...
Text Solution
|
- How are 2 mole NaOH and 2 Molar NaOH different?
Text Solution
|
- Sate Gay Lussac's Law. Calculate the pressure exerted by 4 mole of a...
Text Solution
|
- Draw z vs PO graph for an ideal gas, and CO(2) gas
Text Solution
|
- What is buffer solution? Give one example of acidic buffer solution.
Text Solution
|
- Calculate the solubility of A2xx3 pure water. Assuming that neither ki...
Text Solution
|
- Give equations for the reactions that occur when Sodium metal is dro...
Text Solution
|
- Give equations for the reactions that occur when Magnesium metal is ...
Text Solution
|
- Give equations for the reactions that occur when Sodium peroxide dis...
Text Solution
|
- What happens when limited amount of CO2 gas is passed into milk of lim...
Text Solution
|
- Between -NO(2) and -Br, which one of these is a meta directing group? ...
Text Solution
|
- State anti Merkovnikoff's rule and explain with an example.
Text Solution
|