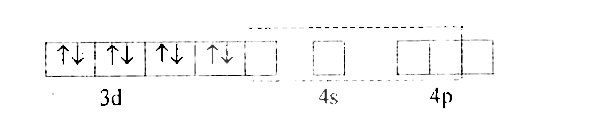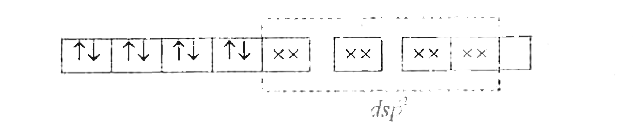Text Solution
Verified by Experts
Topper's Solved these Questions
ANNIUAL EXAM QUESTION PAPER WITH ANSWER (2015)
SUBHASH PUBLICATION|Exercise PART D|12 VideosANNIUAL EXAM QUESTION PAPER WITH ANSWER (2015)
SUBHASH PUBLICATION|Exercise PART E|17 VideosANNIUAL EXAM QUESTION PAPER WITH ANSWER (2015)
SUBHASH PUBLICATION|Exercise PART B|10 VideosAMINES
SUBHASH PUBLICATION|Exercise QUESTIONS|38 VideosANNUAL EXAM QUESTION PAPER MARCH 2017
SUBHASH PUBLICATION|Exercise PART D|11 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-ANNIUAL EXAM QUESTION PAPER WITH ANSWER (2015)-PART C
- Describe the three steps involved in the leaching of bauxite to get pu...
Text Solution
|
- Write the equations involved in the preparation of nitric acid by Ostw...
Text Solution
|
- complete the following equations: CH4+2O2to
Text Solution
|
- complete the following equations: 2Fe^(+3)+SO2+2H2Oto
Text Solution
|
- complete the following equations: C(12)H(22)O(11)overset(conc.H2SO4...
Text Solution
|
- Which is the strongest acid among the hydrogen halides? Give one reaso...
Text Solution
|
- Write the structure of Chloric acid (HClO3)
Text Solution
|
- Give reason (one each) for the following : Transition metal are good...
Text Solution
|
- Give reason (one each) for the following : Second ionisation enthalp...
Text Solution
|
- Give reason (one each) for the following : The spin only magnetic mo...
Text Solution
|
- Write the equations involved in the preparation of potassium dichromat...
Text Solution
|
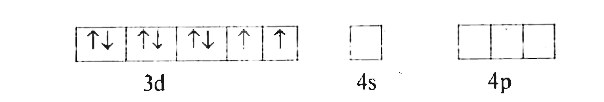
- With the help of Valence Bond theory account for hybridisation, geomet...
Text Solution
|
- For the given complex [Co(NH3)5 Br]SO4, write the IUPAC name and its ...
Text Solution
|
- Which set of d-orbitals of metals ion or atom experience more repulsio...
Text Solution
|