Text Solution
Verified by Experts
The correct Answer is:
JEE ADVANCED PREVIOUS YEAR-JEE (ADVANCED ) 2020-SECTION -III
- 5.00 mL of 0.10 M oxalic acid solution taken in a conical flask is tit...
Text Solution
|
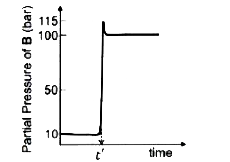
- Consider the reaction AhArr B at 1000 K. At time 't' the temperature o...
Text Solution
|
- Consider a 70% efficient hydrogen - oxygen fuel cell working under sta...
Text Solution
|
- Aluminium reacts with sulfuric acid to form aluminium sulfate and hydr...
Text Solution
|
- ""(92)^(238)U is known to undergo radioactive decay to form ""(82)^(20...
Text Solution
|
- In the following reaction, compound Q is obtained from compound P via ...
Text Solution
|