Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT TELUGU-COORDINATION COMPOUNDS-Exercises
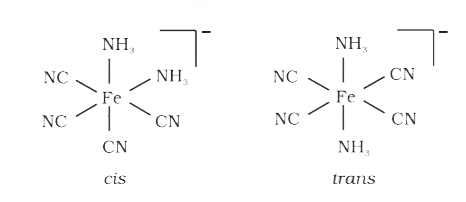
- Draw structures of geometrical isomers of [Fe(NH(3))(2)(CN)(4)]^(-)
Text Solution
|
- Explain the bonding in coordination compounds in terms of Werner’s pos...
Text Solution
|
- FeSO(4) solution mixed with (NH(4))(2)SO(4) solution in 1 : 1 molar ra...
Text Solution
|
- Explain with two examples each of the following: coordination entity, ...
Text Solution
|
- What is meant by unidentate, didentate and ambidentate ligands? Give t...
Text Solution
|
- Specify the oxidation numbers of the metals in the following coordinat...
Text Solution
|
- Using IUPAC norms write the formulas for the Tetrahydroxozincate (II)
Text Solution
|
- Using IUPAC norms write the systematic names of the [Co(NH(3))(6)]Cl(3...
Text Solution
|
- List various types of isomerism possible for coordination compounds, g...
Text Solution
|
- How many geometrical isomers are possible in the following coordinatio...
Text Solution
|
- Draw the structures of optical isomers of: (i) [Cr(C(2)O(4))(3)]^(3-...
Text Solution
|
- Draw all the isomers (geometrical and optical) of : (i) [CoCl(2)(en)...
Text Solution
|
- Write all the geometrical isomers of [Pt(NH(3))(Br)(Cl)(py)] and how m...
Text Solution
|
- Aqueous copper sulphate solution (blue in colour) gives: (i) a green...
Text Solution
|
- What is the coordination entity formed when excess of aqueous KCN is a...
Text Solution
|
- Discuss the nature of bonding and magnetic behaviour in the [Fe(CN)(6)...
Text Solution
|
- Sketch the splitting of d orbitals in an octahedral crystal field,
Text Solution
|
- What is spectrochemical series ? Explain the difference between a weak...
Text Solution
|
- What is crystal field splitting energy? How does the magnitude of Delt...
Text Solution
|
- [Cr(NH(3))(6)]^(3+) is paramagnetic while [Ni(CN)(4))]^(2-) is diamagn...
Text Solution
|
- A solution of [Ni(H(2)O)(6)]^(2+) is green but a solution of [Ni(CN)(4...
Text Solution
|