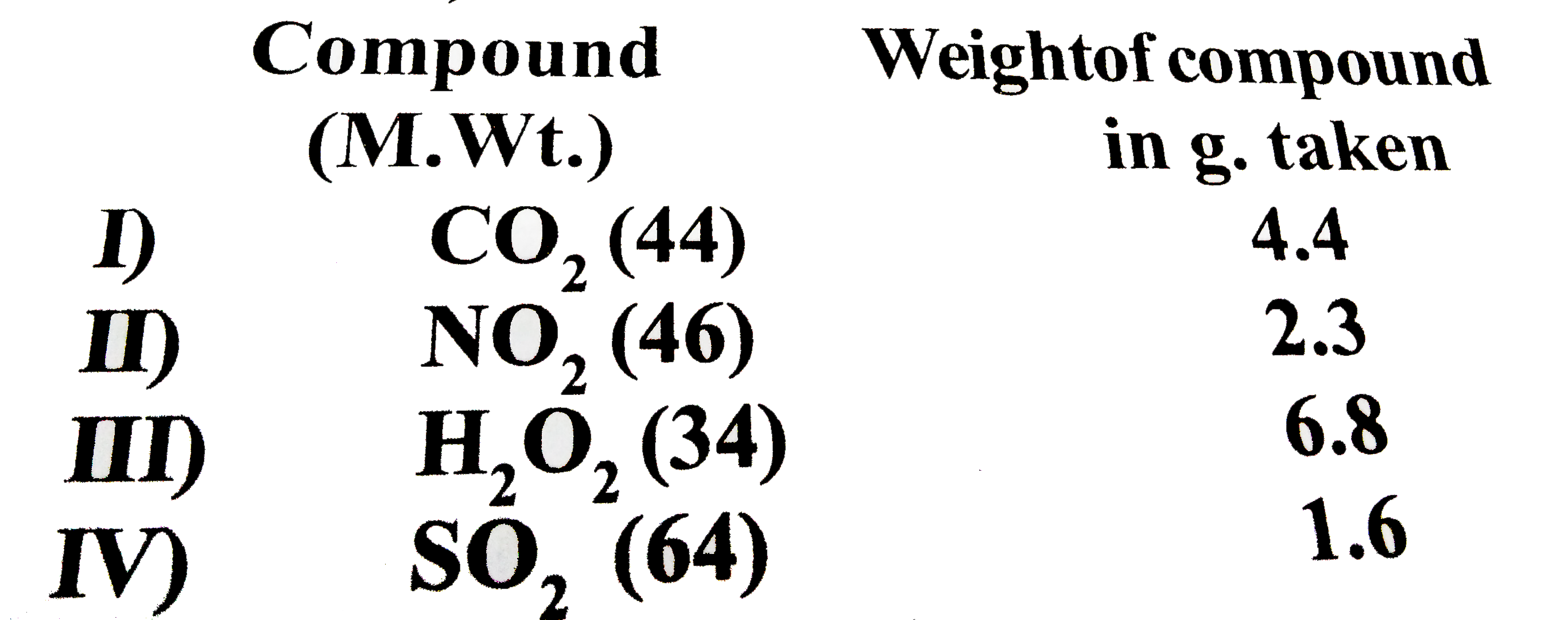A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
NARAYNA|Exercise EXERCISE - II (H.W)(EQUIVALENT WEIGHT)|17 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NARAYNA|Exercise EXERCISE - II (H.W)(PERCENTAGE WEIGHT OF ELEMENTS IN COMPOUNDS MOLECULAR FORMULA & EMPERICAL FORMULA)|8 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NARAYNA|Exercise EXERCISE - II (H.W)(LAWS OF CHEMICAL COMBINATIONS)|14 VideosSOME BASIC CONCEPTS IN CHEMISTRY STOICHIOMETRY (PART-I)
NARAYNA|Exercise All Questions|555 VideosSOME BASIC PRINCIPLES AND TECHNIQUES
NARAYNA|Exercise EXERCISE -IV (QUALITATIVE AND QUANTITATIVE ANALYSIS OF ORGANIC OF COMPOUNDS)|8 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-SOME BASIC CONCEPTS OF CHEMISTRY-EXERCISE - II (H.W)(MOLE CONCEPT ATOMIC MASS, MOLECULAR MASS, AVOGADRO NUMBER)
- The ratio of the mass of C-12 atom to that of an atom of element X (wh...
Text Solution
|
- Study the following table: Which two compounds have least weight of ox...
Text Solution
|
- Which of the following contain 9 xx 10^(23) oxygen atoms?
Text Solution
|
- Number of gram atoms of oxygen present in 0.3 mole of (COOH)(2).2H(2)O...
Text Solution
|
- Ordinary water contain one part of heavy water per 6000 parts by weigh...
Text Solution
|
- No. of moles of water in 488.6 gms of BaCl(2).2H(2)O are (molecular we...
Text Solution
|
- The number of moles of carbon dioxide which contain 8 g of oxygen is
Text Solution
|
- 22.4 litre of water vapour at NTP, When condensed to water occupies an...
Text Solution
|