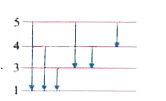
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
NARAYNA|Exercise EXERCISE - II (C. W.) dE-BROGLIE.S & HEISENBERG.S|8 VideosSTRUCTURE OF ATOM
NARAYNA|Exercise EXERCISE - II (C. W.) QUANTUM MECHANICS & NUMBERS|9 VideosSTRUCTURE OF ATOM
NARAYNA|Exercise EXERCISE - II (C. W.) H-SPECTRUM|9 VideosSTATES OF MATTER
NARAYNA|Exercise A & R TYPE QUESTIONS|16 VideosTHERMODYNAMICS
NARAYNA|Exercise Assertion- Reason|5 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-STRUCTURE OF ATOM-EXERCISE - II (C. W.) BOHR.S ATOMIC MODEL
- The velocity of electron in first orbit of H-atom as compared to the v...
Text Solution
|
- In a collection of H-atoms, all the electrons jump from n=5 to ground ...
Text Solution
|
- What is likely to be principal quantum number for a circular orbit of ...
Text Solution
|
- The de-Broglie wavelength of an electron in the first Bohr orbit is
Text Solution
|
- A single electron in an ion has ionization energy equal to 217.6eV. Wh...
Text Solution
|
- The ionisation energy for the Hydrogen atom in the ground state is 2.1...
Text Solution
|
- If the diameter of a carbon atom is 0.15 nm, calculate the number of c...
Text Solution
|
- An electronic transition in hydrogen atom result in the formation of H...
Text Solution
|
- The wavelength of a spectral line emmited by hydrogen atom in the lyma...
Text Solution
|