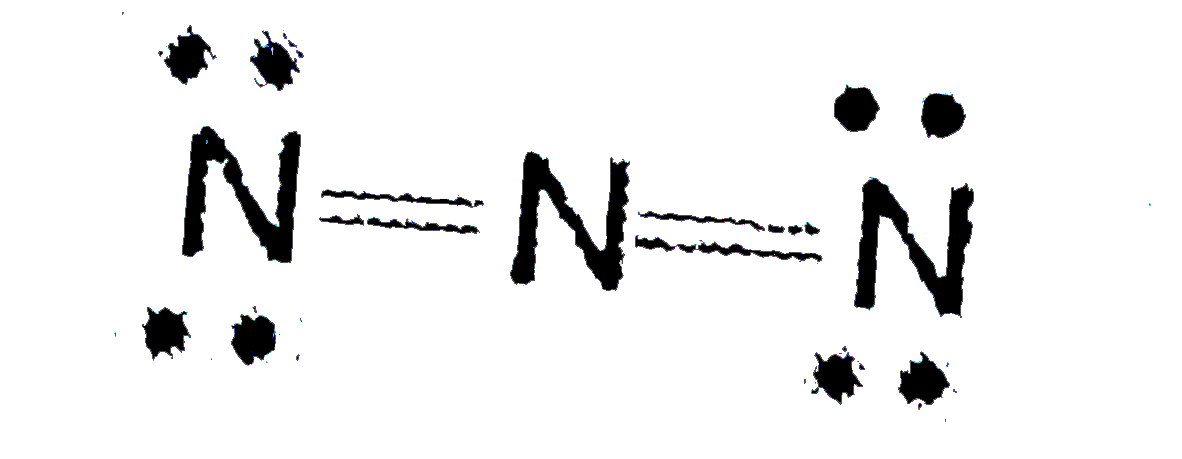
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise CUQ (VALENCE BOND THEORY)|4 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise CUQ (VSEPR THEORY)|8 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise CUQ (POLARITY AND DIPOLE MOMENT)|6 VideosCARBONYL COMPOUNDS
NARAYNA|Exercise LEVEL -VI|99 VideosCHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -IV|33 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-CHEMICAL BONDING AND MOLECULAR STRUCTURE-CUQ (RESONANCE AND FORMAL CHARGE)
- Resonance structures of a molecule do not have:
Text Solution
|
- In which of the following compounds resonance does not occurs (a) H2...
Text Solution
|
- Which of the following Lewis dot structure of CO2 is incorrect ?
Text Solution
|
- In the following electron dot structure, calculate the formal charge f...
Text Solution
|