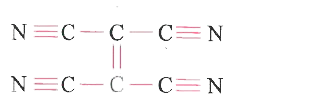
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE- I (C.W.) (DATIVE BOND)|6 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE- I (C.W.) (MOLECULAR ORBITAL THEORY)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE- I (C.W.) (VSEPR THEORY)|6 VideosCARBONYL COMPOUNDS
NARAYNA|Exercise LEVEL -VI|99 VideosCHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -IV|33 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-CHEMICAL BONDING AND MOLECULAR STRUCTURE-EXERCISE- I (C.W.) (HYBRIDISATION)
- The number of hybrid orbitals in a molecule of decane are
Text Solution
|
- The hybridisation of Nitrogen in Nitrate ion is
Text Solution
|
- Hybridisation of iodine hepta fluoride molecule is
Text Solution
|
- A molecule is formed by sp^3d^2 hybridisation. Bond angle in it is :
Text Solution
|
- The molecule which contains sigma(sp^3-sp^3) and sigma(sp^3-p) bonds i...
Text Solution
|
- The C - H bond in propane is
Text Solution
|
- Number of hybrid orbitals present in a molecule of propene are
Text Solution
|
- Hybridization of carbon in C3O2 is :
Text Solution
|
- The type of hybrid orbitals used by the oxygen atom in Cl2O molecule i...
Text Solution
|
- In piperidine , the hybrid state assumed by N is
Text Solution
|
- In which of the following sepcies , is the underlined carbon has sp^3 ...
Text Solution
|
- Carbon atoms in C2(CN)4 are :
Text Solution
|
- H-B- H bond angle in BH4^- is :
Text Solution
|
- A square planar complex is formed by hybridization of which atomic orb...
Text Solution
|