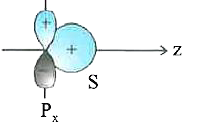
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE- II (C.W.) (VSEPR THEORY)|7 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE- II (C.W.) (HYBRIDISATION)|8 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NARAYNA|Exercise EXERCISE- II (C.W.) (BOND POLARITY AND DIPOLE MOMENT)|8 VideosCARBONYL COMPOUNDS
NARAYNA|Exercise LEVEL -VI|99 VideosCHEMICAL EQUILIBRIUM
NARAYNA|Exercise Exercise -IV|33 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-CHEMICAL BONDING AND MOLECULAR STRUCTURE-EXERCISE- II (C.W.) (VALENCE BOND THEORY)
- Which of the following is a zero overlap which leads to non-bonding?
Text Solution
|
- VALENCE BOND THEORY
Text Solution
|
- The shape of water molecule is same as that of
Text Solution
|
- The strength of bonds by 2s -2s, 2p2p and 2p-2s overlap has the order
Text Solution
|
- For compounds , A : Tetracynoethene B : Carbon dioxide C: Benze...
Text Solution
|
- VALENCE BOND THEORY
Text Solution
|
- According to VBT any covalent bond will be formed by overlapping of at...
Text Solution
|
- The bond between chlorine and bromine in BrCl is
Text Solution
|