A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-CHEMICAL BONDING AND MOLECULAR STRUCTURE-EXERCISE -4
- Arrange LiF, NaF, KF, RbF and CsF in order of increasing lattice energ...
Text Solution
|
- Which of the following are arranged in the decreasing order of dipole ...
Text Solution
|
- The dipole moment of is 1.5 D. The dipole moment of is:
Text Solution
|
- The bond angle in H2S is
Text Solution
|
- If H - X bond length is 2Å and H - X has dipole moment 5.12 xx 10^(-30...
Text Solution
|
- Arrange the following compounds in order of increasing dipole moment ....
Text Solution
|
- Which one of the following arrangements of molecules is correct on the...
Text Solution
|
- Which of the following molecule has highest bond energy ?
Text Solution
|
- In NO(3)^(-) ion, the number of bond pair and lone pair of electrons o...
Text Solution
|
- Among the following the pair in which the two species are not isostruc...
Text Solution
|
- In a regular octahedral molecule MX(6) the number of X - M - X bonds ...
Text Solution
|
- In an octahedral structure , the pair of d orbitals involved in d^(2)...
Text Solution
|
- In correct statement about the structure of PCl5
Text Solution
|
- Which statement is incorrect for OSF4 ?
Text Solution
|
- Which of the following statement is/are true 1) PH5 and BiCl5 donot ...
Text Solution
|
- The states of hybridisation of boron and oxygen atoms in boric acid (H...
Text Solution
|
- The d-orbital involved in sp^3d hybridisation is
Text Solution
|
- Trimethylamine is a pyramidal molecule and formamide is a planar mol...
Text Solution
|
- Which statement is correct about HCHO ?
Text Solution
|
- The correct order of hybridization of the central atom in the followin...
Text Solution
|
 is 1.5 D. The dipole moment of
is 1.5 D. The dipole moment of 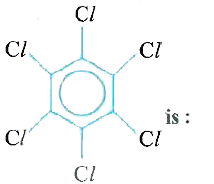 is:
is: