A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
NARAYNA|Exercise WORKED OUT QUESTIONS|2 VideosSTATES OF MATTER
NARAYNA|Exercise EVALUATE YOURSELF - 1|4 VideosSOME BASIC PRINCIPLES AND TECHNIQUES
NARAYNA|Exercise EXERCISE -IV (QUALITATIVE AND QUANTITATIVE ANALYSIS OF ORGANIC OF COMPOUNDS)|8 VideosSTRUCTURE OF ATOM
NARAYNA|Exercise EXERCISE - IV EXEMPLAR PROBLEMS|24 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-STATES OF MATTER-A & R TYPE QUESTIONS
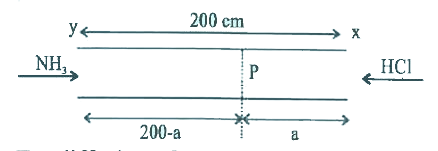
- A straight glass tube as shown, has 2 inleta X and Y at the two ends o...
Text Solution
|
- Assertion (A) : The heat absorbed during the isothermal expansion of a...
Text Solution
|
- Assertion: The value of van der Waals constant a is larger for ammonia...
Text Solution
|
- Assertion: Helium shows only positive deviations from ideal behaviour....
Text Solution
|
- Assertion (A) : CH(4) , CO(2) has value of Z (compressibility factor )...
Text Solution
|
- STATEMENT-1 : The average translational kinetic energy per molecule of...
Text Solution
|
- Assertion: van der Waals equation is applicable only to non-ideal gase...
Text Solution
|
- Assertion:Pressure is exerted by gas in a container with increasing te...
Text Solution
|
- Assertion: Gases do not settle at the bottom of container. Reason: G...
Text Solution
|
- Assertion: A mixture of He and O(2) is used for respiration for deep s...
Text Solution
|
- Assertion (A) : All molecules in a gas have some speed . Reason (R) ...
Text Solution
|
- Assertion: Effusion rate of oxygen is smaller than nitrogen. Reason:...
Text Solution
|
- Assertion: Compressibility factor for hydrogen varies with pressure wi...
Text Solution
|
- Assertion (A) : At high pressure , for one mole of a real gas , the co...
Text Solution
|
- Assertion: Pressure exerted by a mixture of gases is equal to the sum...
Text Solution
|
- Assertion (A) : 22.4 L of nitrogen at S.T.P and 5.6 L of oxygen at S.T...
Text Solution
|
- Assertion: A lighter gas diffuse more rapidly than a heavier gas. Re...
Text Solution
|