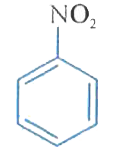
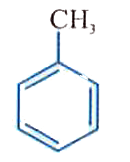
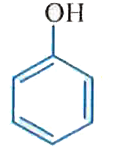
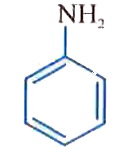
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
NARAYNA|Exercise EXERCISE - 1 (C.W) (OXIDATION AND REDUCTION)|9 VideosHYDROCARBONS
NARAYNA|Exercise EXERCISE - 1 (C.W) (ADDITION REACTION)|5 VideosHYDROCARBONS
NARAYNA|Exercise EXERCISE - 1 (C.W) (CARCINOGENICITY)|3 VideosGENERAL ORGANIC CHEMISTRY
NARAYNA|Exercise Matrix-Match type questions|7 VideosHYDROGEN & ITS COMPOUCDS
NARAYNA|Exercise Interger type questions|10 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-HYDROCARBONS -EXERCISE - 1 (C.W) (ELECTROPHILIC AROMATIC SUBSTITUTION REACTION:)
- Among the following compounds the decreasing order of reactivity towar...
Text Solution
|
- Which of the following structures correspond to the product expected, ...
Text Solution
|
- Which of the following compounds react slower than benzene in electrop...
Text Solution
|
- What is the end product which is obtained on the nitration on toluene?
Text Solution
|
- In Friedel Crafts synthesis of C(6)H(5)-CH(3), reactants in addition o...
Text Solution
|