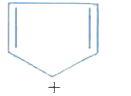
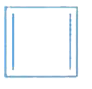
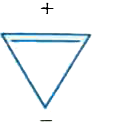
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
NARAYNA|Exercise EXERCISE - 2 (H.W)|34 VideosHYDROCARBONS
NARAYNA|Exercise EXERCISE - 2 (H.W) (ALKENES) (PREPARATIONS OF ALKENES)|2 VideosHYDROCARBONS
NARAYNA|Exercise EXERCISE - 1 (H.W) (DIRECTING INFLUENCE OF FUNCTIONAL GROUPS & CHEMICAL REACTIVITY)|2 VideosGENERAL ORGANIC CHEMISTRY
NARAYNA|Exercise Matrix-Match type questions|7 VideosHYDROGEN & ITS COMPOUCDS
NARAYNA|Exercise Interger type questions|10 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-HYDROCARBONS -EXERCISE - 2 (C.W)
- Using anhydrous AlCl(3) as catalyst, which one of the following reacti...
Text Solution
|
- Reaction of HBr with propene in the presence of peroxide gives :-
Text Solution
|
- Among the following the aromatic compound is
Text Solution
|
- The compound, CH(3)CH(2)-overset(CH(3))overset("| ")"C "=CH-CH(3) on ...
Text Solution
|
- Which alkene on ozonolysis gives CH(3)CH(2)CHO and CH(3)underset(O)und...
Text Solution
|
- Among the following alkenes, (I)1 - butene (II) cis -2- butene (III)...
Text Solution
|
- In Friedel-Crafts reaction for preparation of toluene, the reactants i...
Text Solution
|
- CH(2)=CH-CH(2)-CH-CH(2)overset(NBS)to[A]. The major product [A] is
Text Solution
|
- Na//NH(3)(l) converts hex -3- yne to :
Text Solution
|
- Reactivity order of halides of dehydrohalogenation is
Text Solution
|
- Among the following,the most reactive towards alcoholic KOH is
Text Solution
|
- The case of elimination E(1) increase in the order of alkyl halide as ...
Text Solution
|
- Which of the following will give six isomers in when monochlorinated?
Text Solution
|
- on ozonolysis gives :
Text Solution
|
- Acetylene gives
Text Solution
|
- In the reaction given below, the product C is CaC(2)overset(H(2)O)ra...
Text Solution
|
- When methane is made to react with a halogen (X(2)), halides are forme...
Text Solution
|
- An alkane of mol. Weight 72 gives on monochlorination only one product...
Text Solution
|
- The correct order of boiling point order for corresponding hydrocarbon...
Text Solution
|
- Identify (Y) in the following reaction series C(2)H(5)Ioverset("Alco...
Text Solution
|