A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-CO-ORDINATE COMPOUNDS -Exercise-4
- Mohr's salt on dissociation in water gives
Text Solution
|
- Complex compound is made up of
Text Solution
|
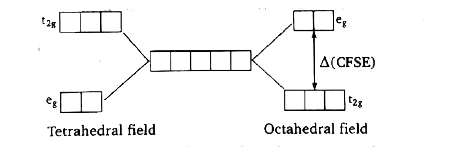
- When degenerate d-orbitals of an isolated atom/ion come under influenc...
Text Solution
|
- The reducing power of the metal decreases in the order:
Text Solution
|
- Which of these species has same number of unpaired electrons with weak...
Text Solution
|
- Why are low spin tetrahedral complexes not formed ?
Text Solution
|
- Value of CFSE, in tetrahedral complex having 3d^4 configuration of met...
Text Solution
|
- The non -existant metal carbonyl among the following is
Text Solution
|
- The pi - bounded organometallic compound which has ethylene as one of ...
Text Solution
|
- If X' is a Anti cancerous drug which of the following conversions will...
Text Solution
|
- Which of the following configuration will not give John- teller distor...
Text Solution
|
- Which of the following does not have a metal carbon bond?
Text Solution
|
- Fe(4)[Fe(CN)(6)](3) a blue coloured complex. Average oxidation number ...
Text Solution
|
- Magnetic moment (spin only) of octahedron complex having SFSE=-0.8Delt...
Text Solution
|
- Given that maximum absorption for d-d transition in [Ti(H2O)(6)]^(3+) ...
Text Solution
|
- The CFSE for octahedral [CoCl(6)]^(4-) is 18,000 cm^(-1). The CFSE for...
Text Solution
|
- Which of the following ion does not exist
Text Solution
|
- Low spin complex of d^6-cation in an octahedral field will have the fo...
Text Solution
|
- Which of the following complex (Werner presentation) have minimum elec...
Text Solution
|
- Which of the following octahedral complexes shown geometrical as well ...
Text Solution
|