Text Solution
Verified by Experts
Topper's Solved these Questions
BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise In Text Questions - Evaluate Yourself|8 VideosBASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise Textual Calculation based on Stoichiometry solved|6 VideosBASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise Additional Questions Solved(5-Mark Questions)|29 VideosALKALI AND ALKALINE EARTH METALS
FULL MARKS|Exercise Additional Questions Solved|224 VideosBASIC CONCEPTS OF ORGANIC REACTIONS
FULL MARKS|Exercise Additional Questions Solved (5-Marks Question)|10 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS -Textual Evaluation Solved(Write brief answer to the following questions.)
- Define relative atomic mass.
Text Solution
|
- What do you understand by the term mole?
Text Solution
|
- Define equivalent mass.
Text Solution
|
- What do you understand by the term oxidation number?
Text Solution
|
- Distinguish between oxidation and reduction
Text Solution
|
- Calculate the molar mass of the following compounds. (i)Urea |CO(N...
Text Solution
|
- The density of carbon dioxide is equal to 1.965 kg m^(-3) at 273 K and...
Text Solution
|
- Which contains the greatest number of moles of oxygen atoms? (i) 1 ...
Text Solution
|
- Calculate the average atomic mass of naturally occurring magnesium usi...
Text Solution
|
- In a traction x+y + z(2)to xyz(2), Identity the limiting reagent If ti...
Text Solution
|
- Mass of one atom of an element is 6.645 xx 10^(-23) g. How many mole's...
Text Solution
|
- What is the difference between molecular mass and molar mass? Calculat...
Text Solution
|
- What is the empirical formula of the following? (i) Fructose (C(6)...
Text Solution
|
- The reaction between aluminium and ferric oxide can generate temperatu...
Text Solution
|
- How many moles of ethane is equaired to produce 44 g of CO(2(g)) after...
Text Solution
|
- Hydrogen peroxide is an oxidising agent. It oxidises ferrous ion to fe...
Text Solution
|
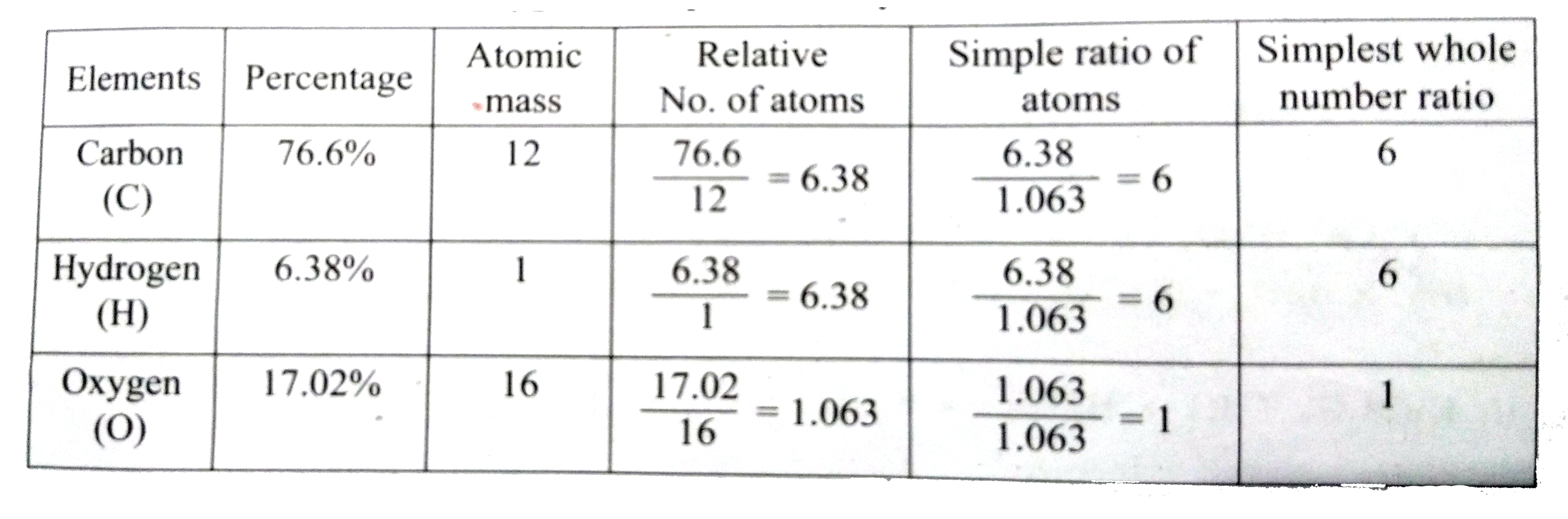
- Calculate the empirical and molecular formula of a compound containing...
Text Solution
|
- A compound on analysis gave the following percentage composition: Na=1...
Text Solution
|
- Balance the following equations by oxidation number method (i) Kr(2...
Text Solution
|
- Balance the following equations by ion electron method. (i) KMnO(4)...
Text Solution
|