Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Additional Questions(5 MARK QUESTIONS)|13 VideosPERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Higher Order Thinking skills (HOTS) Questions|10 VideosPERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Additional Questions(2 MARK QUESTIONS)|46 VideosHYDROGEN
FULL MARKS|Exercise Additional Questions Solved 5-Mark Questions|10 VideosPHYSICAL AND CHEMICAL EQUILIBRIUM
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED (NUMERICAL PROBLEMS)|3 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-PERIODIC CLASSIFICATION OF ELEMENTS -Additional Questions(3 MARK QUESTIONS)
- Why there is a need for classification of elements?
Text Solution
|
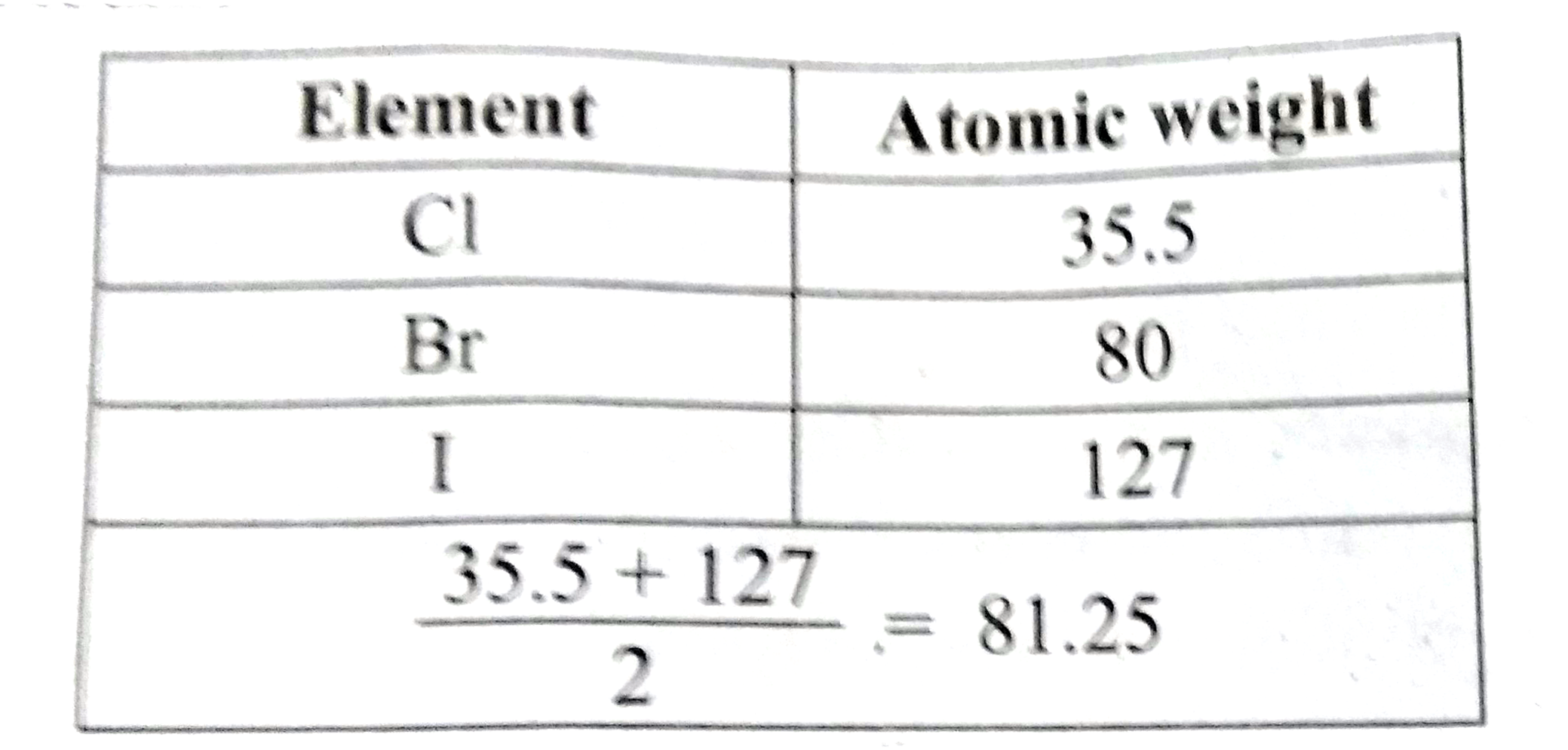
- Prove that the halogens, chlorine, bromine and iodine follow the law o...
Text Solution
|
- What are the salient features of Newland's law of octaves?
Text Solution
|
- How the properties of Eka-silicon was related to germanium?
Text Solution
|
- Compare the properties of Eka-aluminium and gallium.
Text Solution
|
- Explain about the relationship between the atomic number of an element...
Text Solution
|
- What are the reasons behind the Moseley's attempt in finding atomic nu...
Text Solution
|
- Draw a simplified form of periods and elements present in modern perio...
Text Solution
|
- Write the electronic configuration of alkali metals ""(3)Li,(11)Na, ""...
Text Solution
|
- Explain about the classification of elements based on electronic confi...
Text Solution
|
- Write about the electronic configuration of 1^(st) and 2^(nd) period.
Text Solution
|
- How many elements are there in 4^(th) period? Prove it.
Text Solution
|
- How many elements are there in 6^(th) period? Prove it.
Text Solution
|
- What are the two exceptions of block division in the periodic table?
Text Solution
|
- Explain about the salient features of metals.
Text Solution
|
- Explain about the characteristic of non - metals.
Text Solution
|
- Periodic change in electronic configuration is responsible for the phy...
Text Solution
|
- What is covalent radius ? . How would you determine the covalent radiu...
Text Solution
|
- Define metallic radius.
Text Solution
|
- Arrange Na^(+),Mg^(2+) and Al^(3+) in the increasing order of ionic ra...
Text Solution
|