Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Additional Questions(5 MARK QUESTIONS)|13 VideosPERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Higher Order Thinking skills (HOTS) Questions|10 VideosPERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Additional Questions(2 MARK QUESTIONS)|46 VideosHYDROGEN
FULL MARKS|Exercise Additional Questions Solved 5-Mark Questions|10 VideosPHYSICAL AND CHEMICAL EQUILIBRIUM
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED (NUMERICAL PROBLEMS)|3 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-PERIODIC CLASSIFICATION OF ELEMENTS -Additional Questions(3 MARK QUESTIONS)
- Explain about the relationship between the atomic number of an element...
Text Solution
|
- What are the reasons behind the Moseley's attempt in finding atomic nu...
Text Solution
|
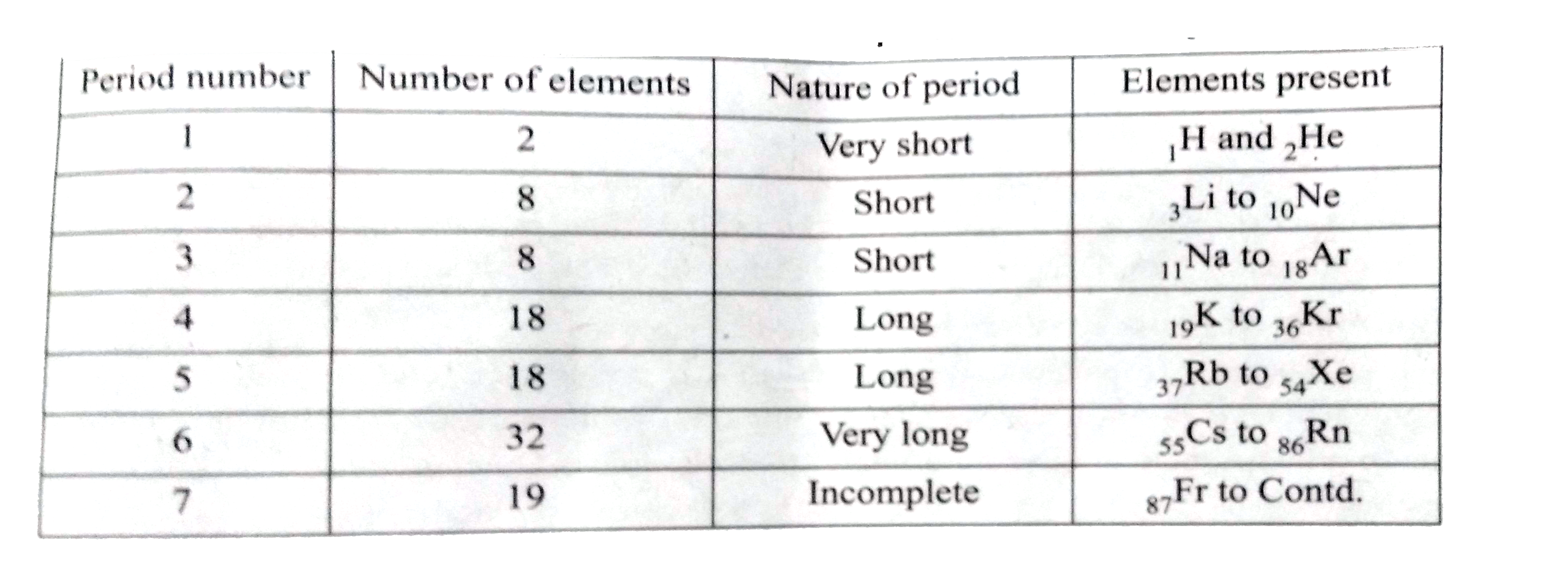
- Draw a simplified form of periods and elements present in modern perio...
Text Solution
|
- Write the electronic configuration of alkali metals ""(3)Li,(11)Na, ""...
Text Solution
|
- Explain about the classification of elements based on electronic confi...
Text Solution
|
- Write about the electronic configuration of 1^(st) and 2^(nd) period.
Text Solution
|
- How many elements are there in 4^(th) period? Prove it.
Text Solution
|
- How many elements are there in 6^(th) period? Prove it.
Text Solution
|
- What are the two exceptions of block division in the periodic table?
Text Solution
|
- Explain about the salient features of metals.
Text Solution
|
- Explain about the characteristic of non - metals.
Text Solution
|
- Periodic change in electronic configuration is responsible for the phy...
Text Solution
|
- What is covalent radius ? . How would you determine the covalent radiu...
Text Solution
|
- Define metallic radius.
Text Solution
|
- Arrange Na^(+),Mg^(2+) and Al^(3+) in the increasing order of ionic ra...
Text Solution
|
- Arrange the ions F^(-),O^(2-) and N^(3-) in the increasing order of th...
Text Solution
|
- Mention some characteristics of ionization energy.
Text Solution
|
- Why ionization energy and electron affinity are calculated in gaseous ...
Text Solution
|
- How does the shielding effect caused by inner electrons affect the ion...
Text Solution
|
- Ionization energy of Mg is greater than that of Al. Why?
Text Solution
|