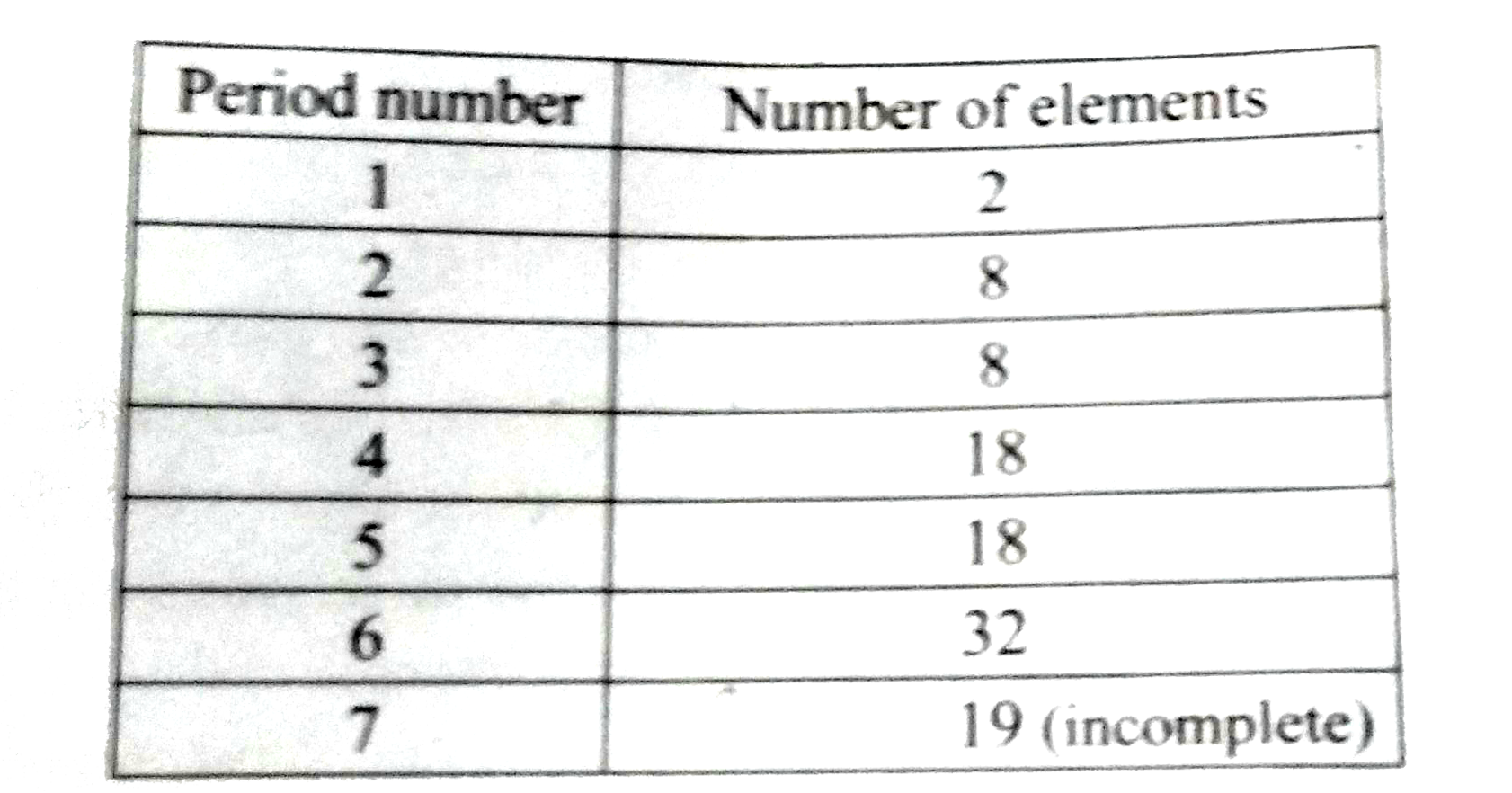
Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Higher Order Thinking skills (HOTS) Questions|10 VideosPERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Activity 3.1|1 VideosPERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Additional Questions(3 MARK QUESTIONS)|39 VideosHYDROGEN
FULL MARKS|Exercise Additional Questions Solved 5-Mark Questions|10 VideosPHYSICAL AND CHEMICAL EQUILIBRIUM
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED (NUMERICAL PROBLEMS)|3 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-PERIODIC CLASSIFICATION OF ELEMENTS -Additional Questions(5 MARK QUESTIONS)
- (a) State Mendeleev's periodic law. (b) Describe about the merits of...
Text Solution
|
- Mention Anomalies of Mendeleev 's periodic table.
Text Solution
|
- Explain about the structural features of Moseley's long form of period...
Text Solution
|
- Explain the merits of Moseley's long form of periodic table.
Text Solution
|
- Explain about the general characteristics of periods.
Text Solution
|
- Explain about the salient features of groups.
Text Solution
|
- Explain the classification of elements based on chemical behavior and ...
Text Solution
|
- (a) Define atomic radius. (b) What are the difficulties in determini...
Text Solution
|
- Prove that the atomic radii is a periodic property.
Text Solution
|
- What are the factors influencing ionization enthalpy.
Text Solution
|
- (a) Define ionization energy. (b) Prove that ionization energy is a ...
Text Solution
|
- Distinguish between electron affinity and electron negativity.
Text Solution
|
- What are the anomalous properties of second period elements?
Text Solution
|