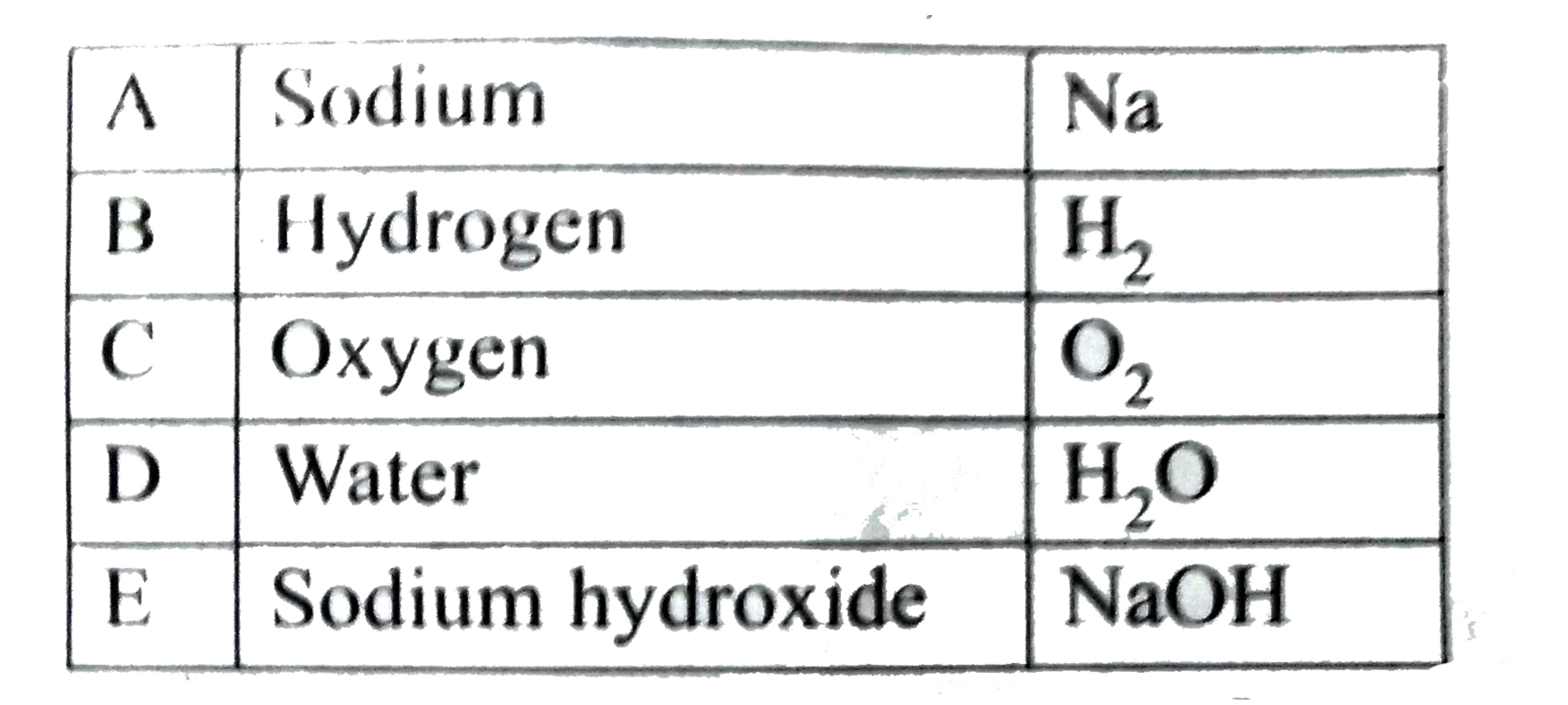
Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROGEN
FULL MARKS|Exercise Additional Questions Solved(I. Choose the correct answer)|42 VideosHYDROGEN
FULL MARKS|Exercise Additional Questions Solved( II. Match the following)|2 VideosHYDROGEN
FULL MARKS|Exercise Additional Questions Solved 5-Mark Questions|10 VideosHYDROCARBONS
FULL MARKS|Exercise Additional Question Solved (5 mark question )|15 VideosPERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Activity 3.2|1 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-HYDROGEN -Textual Evaluation Solved(II. Write brief answer to the following questions:)
- Write the expected formulas for the hydrides of 4^(th) period elements...
Text Solution
|
- Write chemical equation for the following reactions. (1) reaction of h...
Text Solution
|
- Complete the following chemical reactions and classify them into (a) h...
Text Solution
|
- Hydrogen peroxide can function as an oxidising agent as well as reduci...
Text Solution
|
- Do you think that heavy water can be used for drinking purposes?
Text Solution
|
- What is water-gas shift reaction?
Text Solution
|
- Justify the position of hydrogen in the periodic table?
Text Solution
|
- What are isotopes? Write the names of isotopes of hydrogen.
Text Solution
|
- Mention the uses of heavy water .
Text Solution
|
- Explain the exchange reactions of deuterium
Text Solution
|
- How do you convert para hydrogen into ortho hydrogen ?
Text Solution
|
- Mention the uses of deuterium.
Text Solution
|
- Explain preparation of hydrogen using electrolysis.
Text Solution
|
- A groups metal (A) which is present in common salt reacts with (B) to ...
Text Solution
|
- An isotope of hydrogen (A) reacts with diatomic molecule of element wh...
Text Solution
|
- NH3 has exceptionally high melting point and boiling point as compared...
Text Solution
|
- Why interstitial hydrides have a lower density than the parent metal.
Text Solution
|
- How do you expect the metallic hydrides to be useful for hydrogen stor...
Text Solution
|
- Arrange NH3,H2O and HF in the order of increasing magnitude of hydroge...
Text Solution
|
- Compare the structures of H(2)O and H(2)O(2)
Text Solution
|