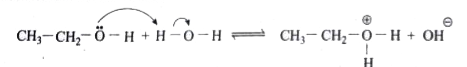Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-SAMPLE PAPER-8-PART - II
- Carbon monoxide is more effective reducing agent than carbon below 983...
Text Solution
|
- Mention the uses of the potash alum.
Text Solution
|
- Why Gd^(3+) is colourless ?
Text Solution
|
- Ionic solids conduct electricity in mollten state but not in solid sta...
Text Solution
|
- What are Lewis acids and bases? Give two example for each.
Text Solution
|
- NH(3),CO(2) are readily adsorbed where as, H(2),N(2) are slowly adsorb...
Text Solution
|
- Alcohol can act as Bronsted base. Prove this statement.
Text Solution
|
- Arrange the following in decresing order of basic strenght
Text Solution
|
- How are biopolymers more beneficial than synthetic polymers?
Text Solution
|