Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-SAMPLE PAPER-8-PART - IV
- (i) How will you purify metals by using iodine ? (ii) Boron does not...
Text Solution
|
- (i) Write the reason for the anomalous behaviour of Nitrogen. (ii) M...
Text Solution
|
- (i) Draw all possible stereo isomers of a complex Ca[Co(NH(3))Cl(Ox)(2...
Text Solution
|
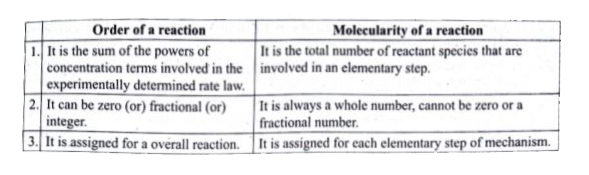
- (i) What is an elementary reaction ? Give the differences between orde...
Text Solution
|
- (i) Derive the relation between pH and pOH (ii) Give three uses of e...
Text Solution
|
- How would you measure the conductivity of ionic solutions ?
Text Solution
|
- (i) What is metamerism ? Give the structure and IUPAC name of metamers...
Text Solution
|
- An organic compound (A) of molecular formula C(7)H(8)O on oxidation wi...
Text Solution
|
- (i) Account for the following : 1. Primary amines (R-NH(2)) have hig...
Text Solution
|
- (i) Why ranitidine is a better antacid than magnesium hydroxide ? (i...
Text Solution
|